What molecule am I?

Nickel samarium oxide (NiSmO3), frequently referred to as samarium nickel oxide, is a synthetic mixed oxide with unusual thermo-optical properties. It and other nickel–rare earth oxides were prepared by Gérard Demazeau and co-workers at the University of Bordeaux (Talence, France) in 1971.
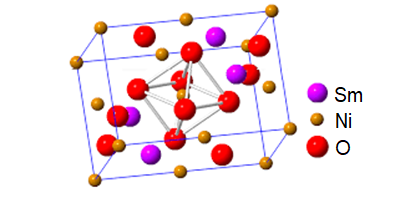
The crystalline oxides have typical ABX3 perovskite structures (see image). Note that the nickel oxidation state is Ni(III).
In recent years, Mikhail A. Kats at the University of Wisconsin–Madison and colleagues there and at four other American research institutions discovered that NiSmO3 appears to violate the venerable Stefan–Boltzmann law, which holds that the energy radiated from a black body is related to the body’s absolute temperature. Specifically, j* = σT4, where
- j* is the total energy radiated per unit surface area of the body per unit time,
- σ is the Stefan–Boltzmann constant, and
- T is the absolute temperature of the body.
Constant σ is related to two other derived physical constants and the speed of light in a vacuum.
The emitted energy is manifested as electromagnetic radiation. In practical terms, as an object is heated, the radiated light becomes brighter. With many substances, including NiSmO3, the radiation is infrared light. But Kats and co-workers found that the IR radiation from NiSmO3 does not brighten with increasing temperature.
Does NiSmO3 really violate the Stefan–Boltzmann law? Not really. As Emily Conover, physics writer for Science News, put it,
According to Kats and colleagues, NiSmO3 is the first known material to exhibit temperature-independent thermal radiation. They state that this phenomenon “has substantial implications for infrared camouflage, privacy shielding, and radiative heat transfer.”
Nickel samarium oxide hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
| Carcinogenicity, category 1 | H350—May cause cancer | |
| Specific target organ toxicity, repeated exposure, category 1 | H372—Causes damage to organs through prolonged or repeated exposure | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
Nickel samarium oxide
fast facts
| CAS Reg. No. | 11132-44-8 |
| SciFinder nomenclature | Nickel samarium oxide (NiSmO3) |
| Empirical formula | NiO3Sm |
| Molar mass | 257.05 g/mol |
| Appearance | Gray crystalline solid |
| Melting point | Not available |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

