What molecule am I?

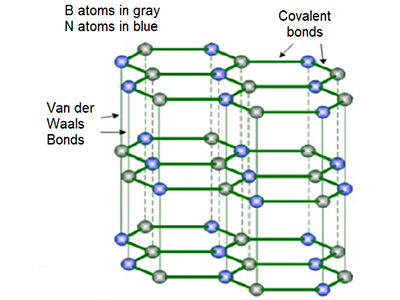
The empirical formula of boron nitride (BN) is deceptive. BN is not at all like other diatomic molecules such as carbon monoxide (CO) and hydrogen chloride (HCl). Rather, it has much in common with carbon, whose representation as the monatomic C is also misleading.
BN, like carbon, has multiple structural forms. BN’s most stable structure, hBN (shown), is isoelectronic with graphite and has the same hexagonal structure with similar softness and lubricant properties. hBN can also be produced in graphene-like sheets that can be formed into nanotubes.
In contrast, cubic BN (cBN) is isoelectronic with diamond. It is not quite as hard, but it is more thermally and chemically stable. It is also much easier to make. Unlike diamond, it is insoluble in metals at high temperatures, making it a useful abrasive and oxidation-resistant metal coating. There is also an amorphous form (aBN), equivalent to amorphous carbon (see below).
BN is primarily a synthetic material, although a naturally occurring deposit has been reported. Attempts to make pure BN date to the early 20th century, but commercially acceptable forms have been produced only in the past 70 years. In a 1958 patent to the Carborundum Company (Lewiston, NY), Kenneth M. Taylor prepared molded shapes of BN by heating boric acid (H3BO3) with a metal salt of an oxyacid such as phosphate in the presence of ammonia to form a BN “mix”, which was then compressed into shape.
Today, similar methods are in use that begin with boric trioxide (B2O3) or H3BO3 and use ammonia or urea as the nitrogen source. All synthetic methods produce a somewhat impure aBN, which is purified and converted to hBN by heating at temperatures higher than used in the synthesis. Similarly, to the preparation of synthetic diamond, hBN is converted to cBN under high pressure and temperature.
Boron nitride hazard information*
| Hazard class** | Hazard statement | |
|---|---|---|
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*As a powder; for solids or platelets, not a hazardous substance or mixture.
**Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Boron nitride
fast facts
| CAS Reg. No. | 10043-11-5 |
| SciFinder nomenclature | Boron nitride (BN) |
| Empirical formula | BN |
| Molar mass | 24.82 g/mol |
| Appearance | Colorless crystals or white powder |
| Melting point | 2973 ºC (subl) |
| Water solubility | Insoluble |
MOTW updates
Hydroxychloroquine was the Molecule of the Week for September 18, 2017. Originally developed as a malaria drug, in 2017 it was being explored as a treatment for patients suffering from the Zika virus.* During the current coronavirus pandemic, dozens of clinical trials are under way to evaluate its efficacy against the SARS-CoV-2 virus.
*The US Food and Drug Administration has not approved hydroxychloroquine as a vaccine or treatment for Zika virus.
Ruxolitinib was one of the Molecules of the Week for March 7, 2016. It is a Janus kinase inhibitor originally used to treat myelofibrosis. In 2016, it was under investigation as a hair-loss preventive. It is now being considered to lessen the effects of COVID-19 by controlling extreme immune responses.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

