What molecule am I?
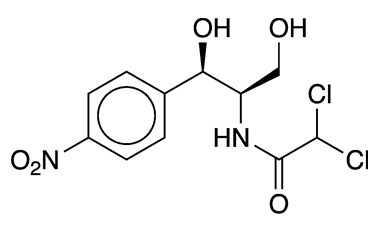
Chloramphenicol is a venerable antibiotic that was isolated from the soil bacterium Streptomyces venezuelae in the late 1940s by researchers at Parke–Davis (Detroit), the University of Illinois (Urbana–Champaign), and Yale University (New Haven, CT). It was originally called chloromycetin.
In July 1949, the Parke–Davis team published the structure of the compound, which then was given the generic name chloramphenicol; Parke–Davis adopted Chloromycetin as its trade name. The group simultaneously announced a laboratory synthesis of chloramphenicol. It was the first totally synthetic antibiotic.
Over the years, chloramphenicol has been used to treat conjunctivitis, meningitis, cholera, and typhoid fever. It is active against bacteria such as Escherichia coli, Staphylococcus spp., and Salmonella spp.
Chloramphenicol can produce some serious adverse effects, including aplastic anemia, neurotoxicity, and some cancers. In 1991, the United States discontinued the use of the oral formulation because of its association with aplastic anemia. In 2007, the World Health Organization classified it as a probable human carcinogen.
Chloramphenicol is still being used in the United States and other countries as an injectable. It is administered intravenously as the more water-soluble prodrug chloramphenicol monosuccinate. Intravenous dosages are greater than oral ones because much of the ester is eliminated before the body can hydrolyze it. Another prodrug, chloramphenicol palmitate, is given orally, but like the parent compound, it is no longer approved for use in the United States.
Chloramphenicol hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Carcinogenicity, category 2 | H351—Suspected of causing cancer | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
MOTW updates
Favipiravir was one of the Molecules of the Week for October 27, 2014. It is an influenza drug that showed promise against Ebola virus disease. Now, its producer, the Fujifilm subsidiary Toyama Chemical (Tokyo), is testing it as a treatment for COVID-19. It is conducting Phase III clinical trials in Japan and Phase II trials in the United States.
Geosmin was the Molecule of the Week for March 23, 2020. It is a natural bacterial product that is found in beet peels, among other plant parts. Yellowfever mosquitoes are attracted to beets, so scientists are experimenting with traps baited with the peels. Geosmin is made by Streptomyces bacteria in beets. Swedish scientists have discovered that tiny invertebrates called springtails are also attracted to the geosmin in beets. They help the bacteria reproduce by spreading their spores into the environment.
Chloramphenicol
fast facts
| CAS Reg. No. | 56-75-7 |
| SciFinder nomenclature | Acetamide, 2, 2-dichloro-N-[(1R,2R)-2-hydroxy-1-(hydroxymethyl)-2-(4-nitrophenyl)ethyl]- |
| Empirical formula | C11H12Cl2N2O5 |
| Molar mass | 323.13 g/mol |
| Appearance | White to pale yellow needles, plates, or powder |
| Melting point | 150.5–151.5 ºC |
| Water solubility | 2.5 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

