What molecule am I?
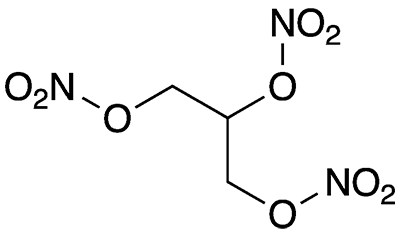
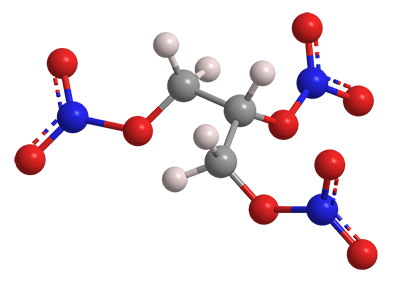
Nitroglycerin, formally 1,2,3-propanetriol trinitrate, is a venerable explosive and, in small doses, a life-saving drug. It was first prepared in 1846 by chemist Ascanio Sobrero at the University of Turin, who was reluctant to publish his work because of the compound’s extreme explosiveness. After numerous accidents, the manufacture and distribution of the pure liquid was soon banned in many jurisdictions.
Recognizing the potential usefulness of nitroglycerin, Swedish chemist and entrepreneur Alfred Nobel sought to make a practical explosive formulation that could be handled reasonably safely. In 1867, he invented dynamite, a mixture of nitroglycerin and sorbents such as diatomaceous earth. He soon developed methods for dynamite manufacture, obtained patents in several countries, and marketed his product. The wealth he amassed allowed him to establish the eponymous prizes in several fields of endeavor.
Soon after he discovered nitroglycerin, Sobrero and others found that tasting the substance caused intense headaches. (In those days, chemists often sampled newly prepared compounds, sometimes with disastrous results.) The headaches suggested that nitroglycerin is a vasodilator; and by the late 1870s, English physician and pharmacologist William Murrell used small doses to alleviate angina pectoris and hypertension.
Today, 140 years later, cardiac patients carry capsules or sprays of dilute nitroglycerin to take sublingually in the event of chest pain or other coronary symptoms. Despite the explosivity and other hazardous properties of pure nitroglycerin (see hazard information table), it is hailed as one of the most important chemical discoveries.
Nitroglycerin hazard information
| GHS classification*: explosives | |
| H200—Unstable explosive | |
| GHS classification: acute toxicity, oral, category 2 | |
| H300—Fatal if swallowed | |
| GHS classification: acute toxicity, dermal, category 1 | |
| H310—Fatal in contact with skin | |
| GHS classification: acute toxicity, inhalation, category 2 | |
| H330—Fatal if inhaled | |
| GHS classification: specific target organ toxicity, repeated exposure, category 2 | |
| H373—Causes damage to organs through prolonged or repeated exposure | |
| GHS classification: hazardous to the aquatic environment, long-term hazard, category 2 | |
| H411—Toxic to aquatic life with long-lasting effects | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Nitroglycerin fast facts
| CAS Reg. No. | 55-63-0 |
| Empirical formula | C3H5N3O9 |
| Molar mass | 227.09 g/mol |
| Appearance | Colorless viscous liquid |
| Boiling point | >50 ºC (explodes) |
| Water solubility | 1.4 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

