What molecule am I?
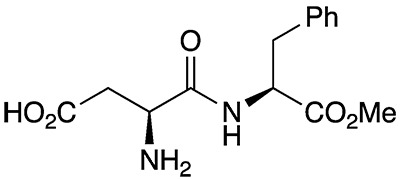
The sweetener aspartame is a dipeptide ester sold under the tradenames Equal and NutraSweet, among others. It is formally a condensation product of aspartic acid with the methyl ester of phenylalanine; but the actual synthetic methods are more complex.
In 1968, a South African patent for synthesizing aspartame and other dipeptide sweeteners was awarded to James M. Schlatter and assigned to G. D. Searle (Chicago), now part of Pfizer. The equivalent US Patent (3,492,131) was awarded in 1970. But not until 1981 did the US Food and Drug Administration approve it for use in foods.
At one time, rumors held that aspartame is carcinogenic; but studies performed from 2006 through 2015 refuted this allegation. The FDA and National Cancer Institute supported these studies.
Aspartame’s sweetness is ≈200 times greater than that of sucrose. Its solubility and stability in aqueous media depend strongly on pH. It is most stable at pH 4.3, which makes it ideal for sweetening carbonated beverages. It is unstable at normal cooking and baking temperatures; but it can be used in “no-heat” recipes.
Aspartame is considered to mimic the qualities of sucrose (e.g., flavor, taste duration, and low aftertaste) better than other artificial sweeteners.
Aspartame hazard information
| GHS classification*: not a hazardous substance or mixture |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Aspartame fast facts
| CAS Reg. No. | 22839-47-0 |
| Empirical formula | C14H18N2O5 |
| Molar mass | 294.31 g/mol |
| Appearance | White crystalline powder or colorless needles |
| Melting point | 246–250 ºC |
| Water solubility | 10 g/La |
a. At pH 7.
MOTW update:
January 23, 2023
Aspartame1 is a widely used dipeptide artificial sweetener that has come under scrutiny because consuming it has been linked to increased appetite and weight gain. It may also be a public health hazard: This month, Xing-Fang Li and co-workers at the University of Alberta (Edmonton) described reactions of aspartame with residual chlorine in tap water to form chlorinated disinfection byproducts—in particular, significant amounts of potentially toxic 2,6-dichloro-1,4-benzoquinone2.
1. CAS Reg. No. 22839-47-0.
2. CAS Reg. No. 697-91-6.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

