What molecules are we?
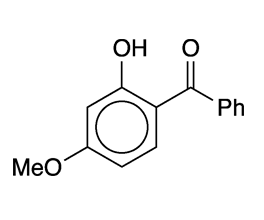
Oxybenzone (Figure 1), formally 2-hydroxy-4-methoxybenzophenone, is an old-line sunscreen ingredient developed in the 1950s by the now-defunct General Aniline & Film (GAF) Corp. It is marketed today under several tradenames. The compound was first synthesized in 1906 in Germany by chemists B. König and Stanisław Kostanecki.
The US Food and Drug Administration approved the use of oxybenzone in sunscreen products in the 1980s. In 2013, a revised approval allowed concentrations of up to 6%. Oxybenzone absorbs light in the UV-B (280–315 nm) and short-wavelength UV-A (315–355 nm) ranges.
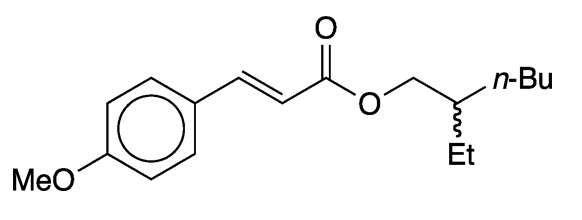
Octinoxate, or octyl methoxycinnamate (Figure 2), is a more recent sunscreen component. It is marketed by Merck under the tradename Eusolex 2292 and by BASF as Uvinul MC80. It absorbs primarily in the UV-B range.
Both compounds are widely used in sunscreens, but this past May, the Hawaii legislature passed a bill that outlaws products that contain them, effective January 2021. The bill is based on studies that show that oxybenzone may harm coral larvae and that both compounds may “bleach” coral, causing it to lose symbiotic algae.
Sunscreen makers, of course, deny that the studies are valid. Nevertheless, Hawaii governor David Ige signed the bill into law earlier this month.
Oxybenzone hazard information
| GHS classification*: skin irritation, category 2 | |
| H315—Causes skin irritation | |
| GHS classification: serious eye irritation, category 2A | |
| H319—Causes serious eye irritation | |
| GHS classification: specific target organ toxicity, single exposure; respiratory tract irritation, category 3 | |
| H335—May cause respiratory irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Octinoxate hazard information
| GHS classification: not a hazardous substance |
Oxybenzone fast facts
| CAS Reg. No. | 131-57-7 |
| Molar mass | 228.24 g/mol |
| Empirical formula | C14H12O3 |
| Appearance | White to pale yellow crystals or powder |
| Melting point | 65.5 ºC |
| Boiling point | 150–160 ºC (5 torr) |
| Water solubility | 13 mg/L |
Octinoxate fast facts
| CAS Reg. No. | 5466-77-3 |
| Molar mass | 290.40 g/mol |
| Empirical formula | C18H26O3 |
| Appearance | Colorless to pale yellow viscous liquid |
| Boiling point | 198–200 ºC (3 torr) |
| Water solubility | <1 mg/L |
MOTW update
Fluralaner was the Molecule of the Week for March 26, 2018. It is a popular flea medication for dogs that needs to be administered at only 12-week intervals. Now, fluralaner and its cousin flea protectant afoxolaner might be used as an oral medication to kill disease-spreading mosquitoes when they bite treated people. A modeling study by researchers at TropIQ Health Sciences (Nijmegen, The Netherlands) and California Institute for Biomedical Research (La Jolla) found that in areas at risk, giving people these drugs would substantially reduce incidences of malaria and Zika. The drugs kill carrier mosquitoes via an attack on an insect-specific ion channel.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

