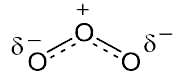

Ozone (O3) plays an extremely important role in the upper atmosphere (stratosphere) and lower atmosphere (troposphere). In the stratosphere (10–50 km above sea level), UV radiation breaks O2 molecules into atomic oxygen (O); O2 and O then recombine to form O3. This first step absorbs almost all UV-C radiation (200–280 nm) from the sun before it passes below ~40 km above sea level; this is the rate-limiting step in O3 formation.
O3 is photolyzed by UV-B radiation (280–320 nm) back to O and O2. This process absorbs a large amount of UV-B radiation, but some still reaches Earth’s surface where it is the primary cause of sunburn. With depleted amounts of O3 in the atmosphere, more UV-B radiation reaches sea level.
A significant amount of Earth’s O3 (~10%) exists in the troposphere (~0–10 km). Here it is not produced by the photolysis of O2 because the required UV-C radiation has been absorbed in the stratosphere. Instead, O3 is produced primarily during the oxidation of CH4 and NO. Anthropogenic generation of these compounds has greatly increased over the past century and led to an increase in tropospheric O3, which is linked to health problems such as asthma.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

