What molecule am I?
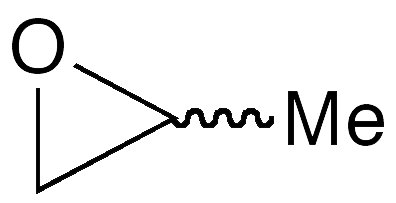
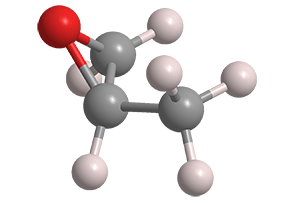
Propylene oxide (PO), formally 2-methyloxirane, is low-boiling liquid that is useful for making many commercial materials. It was known as long ago as 1866, when Eduard Linnemann at the University of Lemberg (Germany) described the conversion of PO to acetone.
The initial method for producing PO was the chlorination of propylene in water to give a mixture of chlorohydrins, followed by dehydrochlorinaton with potassium hydroxide. A process developed subsequently was the direct oxidation of propylene with an organic hydroperoxide. Both processes are in use today.
About two-thirds of worldwide PO production (≈13 million t/year) is used to make polyether polyols, which play an important role in the manufacture of the versatile polyurethane foam. Another ≈20% is hydrolyzed to propylene glycol. As an industrial epoxide, PO is second in importance only to ethylene oxide. PO is a chiral molecule, but almost all of it is produced as the racemic mixture.
As shown in the hazard information table, exposure to PO can cause a wide range of health and environmental problems, some severe. It must be handled with extreme care. .
Propylene oxide hazard information
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 1 | H224—Extremely flammable liquid and vapor | |
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Acute toxicity, dermal, category 3 | H311—Toxic in contact with skin | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Sensitization, skin, category 1 | H317—May cause an allergic skin reaction | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 3 | H331—Toxic if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Specific target organ toxicity, single exposure, narcotic effects, category 3 | H336—May cause drowsiness or dizziness | |
| Germ cell mutagenicity, category 1B | H340—May cause genetic defects | |
| Carcinogenicity, category 1B | H350—May cause cancer | |
| Germ cell mutagenicity, category 1B | H361—Suspected of damaging fertility or the unborn child | |
| Short-term (acute) aquatic hazard, category 3 | H402—Harmful to aquatic life | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Propylene oxide fast facts
| CAS Reg. No. | 75-56-9 |
| SciFinder nomenclature | Oxirane, 2-methyl- |
| Empirical formula | C3H6O |
| Molar mass | 58.08 g/mol |
| Appearance | Colorless liquid |
| Melting point | 34 °C |
| Water solubility | 425 g/L (20 °C) |
MOTW updates
L-Theanine1, the Molecule of the Week for June 15, 2009, is a natural amino acid found in tea leaves and a species of mushroom. It is purported to have beneficial psychological effects; but this past month, Wei Xu, Wen-Jun Xiao, and co-workers at Hunan Agricultural University (Changsha, China) described a potentially more significant medical breakthrough. They found that L-theanine modulates intestine-specific immunity in mice, which, if it acts similarly in humans, can lead to treatments for combating severe allergies caused by ovalbumin, the main protein in egg whites.
Musk ketone2 was the Molecule of the Week for May 8. 2017. It is one of several natural and synthetic “musk” compounds used in the cosmetics industry. In November, Keiichi Yoshikawa and colleagues at Kao Corp. (Haga, Japan) and Duke University (Durham, NC) reported that four other “musk” molecules (musk xylene, mucsone, galaxolide, and helvetolide3), with diverse chemical structures, all activate the same olfactory receptor in humans. Their findings could help develop a unifying theory of musk odors.
1. CAS Reg. No. 3081-61-6.
2. CAS Reg. No. 81-14-1.
3. CAS Reg. Nos. 81-15-2, 10403-00-6, 1222-05-5, and 141773-73-1, respectively.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

