What molecule am I?
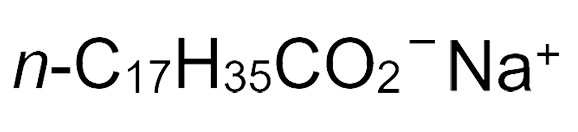
Humans began to use cleaning substances that resemble modern soaps almost five millennia ago. Early crude soaps were made from natural fats and oils and available alkaline materials such as wood ashes. During the Industrial Revolution, manufacturers began to make more refined soaps from purified fatty acids and alkalis such as lye (sodium or potassium hydroxide), quicklime (calcium oxide), or slaked lime (calcium hydroxide).
Sodium stearate is the most common fatty acid salt in today’s soaps. Common sources of the starting material, stearic acid, are vegetable triglycerides obtained from coconut and palm oils and animal triglycerides from tallow. The names stearic and stearate are derived from stéar, the Greek word for tallow.
Besides being a major soap component, sodium stearate is used as an additive in other cosmetic products to form solid “stick” shapes. According to Acme-Hardesty, a Blue Bell, PA–based manufacturer of biobased products, sodium stearate has a wide range of additional uses, including
- emulsifier and dispersant in latex paints;
- ink thickener;
- stabilizer, viscosity enhancer, and dispersant for liquid makeups;
- FDA-approved flavor additive;
- viscosity modifier in gelled fragrances;
- lubricant in polycarbonates and nylons; and
- lubricant and de-dusting agent in rubber production.
Many Web sites provide recipes for making soap at home. But all scratch recipes require lye, which is better off being handled in the lab. If you are willing not to make soap from scratch, you can purchase a “melt and pour” soap, in which the desired oil or fat is already treated with lye. Happy soapmaking!
Sodium stearate hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Hazardous to the aquatic environment, acute hazard, category 2 | H401—Toxic to aquatic life | |
| Hazardous to the aquatic environment, long-term hazard, category 2 | H411—Toxic to aquatic life with long-lasting effects | |
| None | May form combustible dust concentrations in air | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule that you would like us to consider, please send us a message, preferably including links to references about your suggested molecule. And thank you for your interest in Molecule of the Week! —Ed.
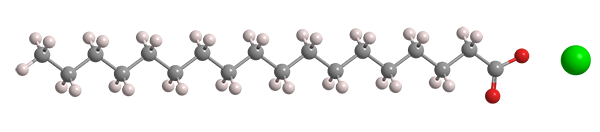
Sodium stearate
fast facts
| CAS Reg. No. | 822-16-2 |
| SciFinder nomenclature | Octadecanoic acid, sodium salt |
| Empirical formula | C18H35NaO2 |
| Molar mass | 306.47 g/mol |
| Appearance | White solid or powder |
| Melting range | 245–270 ºC |
| Water solubility | Soluble, not quantified |
MOTW update
Hinokitiol was the Molecule of the Week for September 4, 2017. It is a tropolone monoterpenoid that shows promise as a drug that can regulate iron transport in animals. More recently, Inga Wessels and colleagues at RWTH Aachen University (Germany) showed that zinc supplementation may inhibit COVID-19 viral replication and that zinc ionophores such as hinokitiol may play a role in this process. An ionophore is a compound that binds ions and allows them to be transported through hydrophobic media such as cell membranes.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.


