What molecule am I?
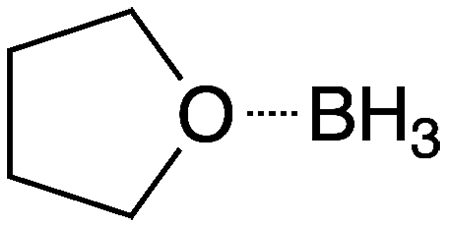
Borane–tetrahydrofuran (BH3–THF) is a charge-transfer complex that is a useful surrogate for diborane1 in organic synthesis. It can be used to reduce carboxylic acids to alcohols or nitriles to primary amines. It reacts with olefins to add the BH2 functional group. Alkyl- or arylboranes formed in this way can further react with unsaturated compounds such as olefins, imines, ketones, and alkynes (the hydroboration reaction) to make useful boron-containing intermediates.
Charge-transfer complexes such as BH3–THF form when a Lewis base (THF in this case) “donates” its unshared electron pair to an electron-deficient Lewis acid such as borane. The electrostatic attraction between the components is not as strong as a covalent bond; but in many cases it is sufficient to make the complex behave as a covalent compound. As an example, BH3–THF can be distilled without decomposing.
BH3–THF’s relative stability makes it a convenient way to use extremely toxic and flammable diborane. But it is not without its own risks. In particular, it reacts violently with water to produce copious amounts of hydrogen. As a result, BH3–THF is normally sold as a 1 M (≈10 wt%) solution in THF. It is available from numerous chemical supply houses worldwide.
1. Under standard conditions, borane dimerizes to produce the more electronically stable diborane.
Borane–tetrahydrofuran hazard information*
| Hazard class* | Hazard statement | |
|---|---|---|
| Flammable liquids, category 2 | H225—Highly flammable liquid and vapor | |
| Chemicals which, in contact with water, emit flammable gases, category 1 | H260—In contact with water releases flammable gases that may ignite spontaneously | |
| Acute toxicity, oral , category 4 | H302—Harmful if swallowed | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Specific target organ toxicity, single exposure, respiratory system, category 3 | H335—May cause respiratory irritation | |
Carcinogenicity, category 2 | H351—Suspected of causing cancer | |
*Hazard information is available only for the article of commerce, typically a 1 M solution in THF. **Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Borane–tetrahydrofuran fast facts
| CAS Reg. No. | 14044-65-6 |
| SciFinder nomenclature | Boron, trihydro (tetrahydro furan)-, (T-4)- |
| Empirical formula | C4H11BO |
| Molar mass | 85.94 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 65–67 ºC |
| Water solubility | Reacts violently |
MOTW update
Pigment violet (PV29) was the Molecule of the Week for February 4, 2019. It is a perylene-based compound that is used in a wide range of coloring applications, including paints, inks, paper, carpets, solar cells, and drugs. In 2018, the US Environmental Protection Agency reported that it was a low risk to human health and the environment, but this was disputed by a scientific advisory committee and environmental groups. In the past month, EPA revised its assessment, stating that PV29 exposure poses unreasonable risks to workers under certain conditions. The agency ordered manufacturer Sun Chemical and importer BASF to provide additional data concerning worker exposure.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

