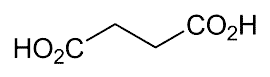
Succinic acid, or butanedioic acid, was first isolated as long ago as 1546 as a distillate from amber. It also occurs naturally in fungi and lichens, and it is a component of the citric acid cycle. Its chief reactions are oxidation to produce fumaric acid and condensation with ketones to produce unsaturated diacids. The commercialization of a biobased process for making succinic acid was announced in January.
MOTW update: April 01, 2019
At the time Succinic acid was the Molecule of the Week, it was considered to be an important biobased chemical; and several projects were under way to produce it from agricultural byproducts. But this past week, the last biosuccinic acid joint venture was dissolved; and the plants built a decade ago to make the product are idle.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

