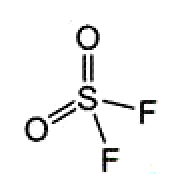
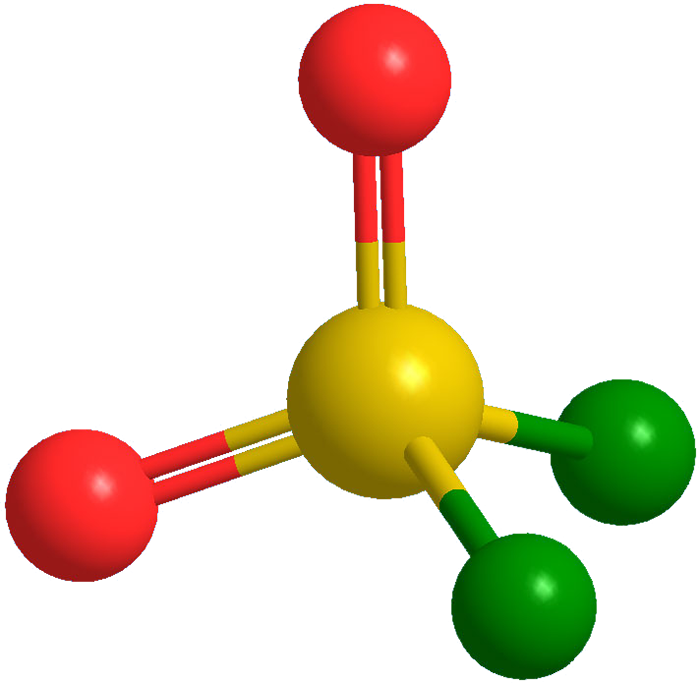
Sulfuryl fluoride was first reported by M. Trautz and K. Ehrmann in 1935. It is relatively unreactive for a sulfur–fluorine compound, but it is very irritating to the respiratory tract. It is primarily used as a fumigant, especially for ridding structures of termites. SO2F2 is 4,000 times more powerful as a greenhouse gas than CO2, and it was recently shown that its persistence in the atmosphere is much longer than previously suspected.
MOTW update:
November 14, 2022
Sulfuryl fluoride1 (SO2F2) is a relatively unreactive gas that is commonly used as a fumigant for controlling termites. It is also a greenhouse gas 4800 times more potent than CO2 with high atmospheric persistence. In October, two environmental organizations filed a legal petition with the California Air Resources Board to phase out fumigants that contain SO2F2 because of the chemical’s strong ability to trap heat in the atmosphere.
1. CAS Reg. No. 2699-79-8.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

