What molecule am I?
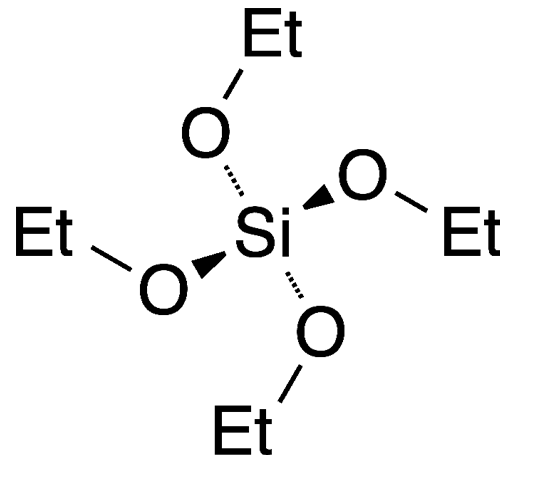
Tetraethyl orthosilicate (TEOS) is an ester of orthosilicic acid, which exists in small amounts in nature wherever silica is in contact with water. The ester is also known by several other names, including ethyl silicate (which is somewhat ambiguous), silicon tetraethoxide, and tetraethoxysilane.
In a landmark paper published in 1928, A. W. Dearing and E. Emmet Reid* at Johns Hopkins University (Baltimore) reported improved syntheses of various alkyl orthosilicates. TEOS was obtained in 70% yield by slowly adding silicon tetrachloride (SiCl4) to cold anhydrous ethanol, followed by removing byproduct hydrogen chloride with a stream of dry air. The reaction system must be rigorously free of water because even though TEOS is only slightly soluble in water, it hydrolyzes to form silica and ethanol. The reaction is the basis of modern TEOS production.
TEOS has multiple specialty uses, including stone hardening (which arrests the decay of structures and art objects), mortar and cement manufacture, and cross-linking silicone polymers. Its 2020 global market is valued at US$245 million for an estimated production of ≈120,000 t.
Tetraethyl orthosilicate hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Flammable liquids, category 3 | H226—Flammable liquid and vapor | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 3 | H331—Toxic if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
MOTW update
Scopolamine and its biochemical precursor hyoscyamine were the Molecules for the Week of October 21, 2013. Both are highly toxic and hallucinogenic. They are found in plants of the Solanaceae family such as deadly nightshade, mandrake, jimsonweed, and—surprisingly—tomato. Despite their hazardous nature, both alkaloids are used in medicine. Recently, Christina D. Smolke* and Prashanth Srinivasan at Stanford University (CA) engineered Saccharomyces cerevisiae (brewer’s yeast) to produce hyoscyamine and scopolamine. The biosynthetic pathway is considered to be the longest and most complex to date for engineered yeast.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Tetraethyl orthosilicate fast facts
| CAS Reg. No. | 78-10-4 |
| SciFinder nomenclature | Silicic acid (H4SiO4), tetraethyl ester |
| Empirical formula | C8H20O4Si |
| Molar mass | 208.33 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 169 ºC |
| Water solubility | 1.5 g/L (dec.) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

