What molecule am I?
White phosphorus is one of three allotropes of the element phosphorus. The other two are red, an amorphous polymer, and black, a graphitelike polymer. The substance known as yellow phosphorus is actually white phosphorus that contains impurities (e.g., red phosphorus) or that has darkened from exposure to light. Red phosphorus turns violet or purple when it is heated to >550 ºC.
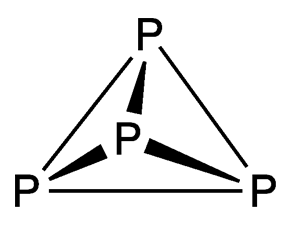
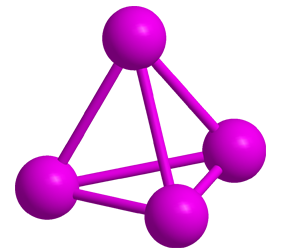
White phosphorus (see images) contains four phosphorus atoms in a tetrahedral arrangement. It has an unpleasant, garliclike odor and is extremely toxic (see hazard information table). It is unstable in air—first forming white fumes before bursting into flames. White phosphorus has been called the “devil’s element” because it glows green in the dark and is pyrophoric.
Because of its instability, white phosphorus is typically stored under water, in which it is barely soluble. The allotrope is soluble in hydrocarbons, carbon disulfide, sulfur chloride (S2Cl2), and other nonpolar solvents.
Molecular phosphorus does not exist in nature; but it is contained in many minerals, primarily hydroxyapatite, fluorapatite, and chorapatite. White phosphorus was discovered in 1669 by Hamburg, Germany, pharmacist/alchemist Hennig Brandt (in some accounts, Brand), who is the subject of the painting The Alchemist Discovering Phosphorus by Joseph Wright. During his search for the mythical philosopher’s stone, Brandt serendipitously produced phosphorus by heating phosphate-containing urine solids with carbonaceous substances. Phosphorus was emitted as a gas (P2), which condensed as a glowing wax.
Brandt did not have an understanding of elements as we know them today. More than 100 years after his discovery, legendary French chemist Antoine Lavoisier recognized phosphorus as an element.
Brandt’s crude process is the basis of modern white phosphorus production. Apatites are mixed with silica (sand) and a carbon source such as coke to produce P2 vapor, which is condensed in water. If fluorapatite is used, the calcium fluoride byproduct can be used to produce fluorine gas.
Worldwide, ≈900,000 t of phosphorus is produced annually. Most of it, ironically, is oxidized back to phosphates for fertilizers (by far the largest use) and the manufacture of pesticides, plasticizers, and animal food additives. The use of white phosphorus itself is limited to ingredients in metallurgy and rodent poisons. It was used as a component of friction matches, until around the turn of the 20th century, when it was replaced by the safer phosphorus sesquisulfide (P4S3).
White phosphorus hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Pyrophoric solids, category 1 | H250—Catches fire spontaneously if exposed to air | |
| Acute toxicity, oral, category 1 | H300—Fatal if swallowed | |
| Skin corrosion/irritation, category 1A | H314—Causes severe skin burns and eye damage | |
| Acute toxicity,inhalation, category 1 | H330—Fatal if inhaled | |
| Hazardous to the aquatic environment, acute hazard, category 1 | H400—Very toxic to aquatic life | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
White phosphorus
fast facts
| CAS Reg. No. | 12185-10-3 |
| SciFinder nomenclature | Phosphorus, mol. (P4) |
| Empirical formula | P4 |
| Molar mass | 123.90 g/mol |
| Appearance | White, waxy crystalline solid |
| Melting point | 44.15 ºC |
| Water solubility | ≈3 mg/L |
MOTW update:
August 25, 2025
White phosphorus1 (P4) is one of the three allotropes of phosphorus. It is toxic and spontaneously inflammable in air.
Because of its hazards, P4 is not commercially available. Last month, Christine M. Thomas*, Tianze Song, and Joshua E. Goldberger* at Ohio State University (Columbus) reported a convenient method for synthesizing P4 on a gram scale. They prepared the compound by heating readily available polymeric red phosphorus2 in a sealed quartz tube to ≈480 °C over 24 h.
1. CAS Reg. No. 12185-10-3.
2. CAS Reg. No. 7723-14-0.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.


