What molecule am I?

George Olah was a Nobel Prize–winning chemist at Case Western Reserve University (Cleveland) and the University of Southern California (Los Angeles) who passed away in March 2017. He was awarded the prize for his research on carbocations—stable organic cations—that he generated with the aid of superacids. Developing superacids was as significant as the carbocation studies.
Superacids have Hammett acidity values (H0) that are greater negative numbers than the –12 for 100% sulfuric acid. James Bryant Conant at Harvard University coined the term in 1927, when he prepared “magic acid” (SbF5–FSO3H) with H0 = –19.2. The strongest superacid to date is fluoroantimonic acid (SbF6–H2F+) with H0 = –31.3.
Traditional superacids that contain elements such as antimony and fluorine are toxic and difficult to handle, so chemist have developed a series of C–H superacids that contain trifluoromethanesulfonyl (triflyl, Tf, or CF3SO2) groups to produce their acidity. The strongest of these is tritriflylmethane (Tf3CH), with a pKa (similar to H0) value of ≈–16.4, depending on the solvent used. C–H superacids are widely used as organic catalysts in C–C bond forming reactions such as Friedel–Crafts acylation and Mukaiyama aldol addition.
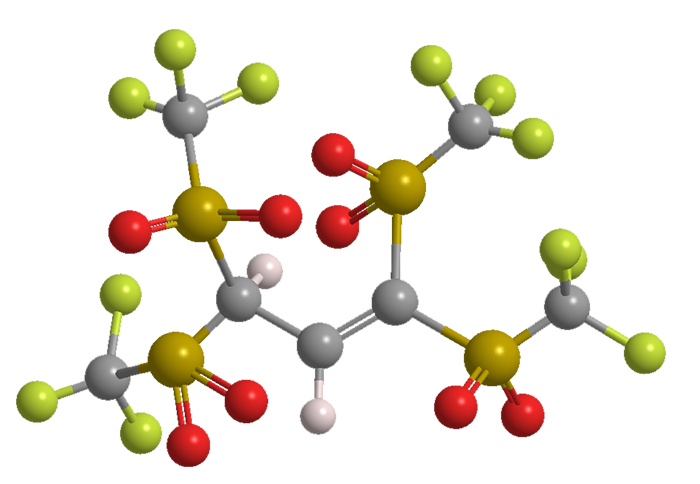
Benjamin List and colleagues at the Max Planck Institute for Coal Research (Mülheim an der Ruhr, Germany) postulated that C–H acids with more electron-withdrawing triflyl groups than Tf3CH might be even better catalysts. They synthesized 1,1,3,3-tetratriflylpropene (TTP), a four-Tf molecule that, when ionized, delocalizes the negative charge throughout the anion.
Although the pKa value of TTP (–15.2) is no stronger than that of Tf3CH, it has better catalytic activity in classical reactions such as Friedel–Crafts. The authors continue to expand the scope of reactions for which TTP is useful; and they believe that “TTP may be useful for applications beyond organocatalysis, such as in electrolytes and ionic liquids, and as a weakly coordinating anion.”

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

