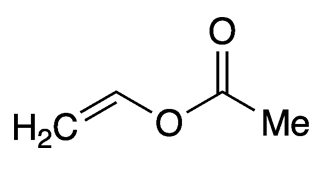
Vinyl acetate synthesis and current production
Esters are usually synthesized from an alcohol and an acid, most frequently, a carboxylic acid. In some cases, an epoxide is used instead of the alcohol; or an acid halide or anhydride replaces the acid. Less often, an alkyl or aryl halide is treated with a carboxylate salt.
But these methods don’t work in the case of vinyl acetate and other vinyl esters because vinyl alcohols aren’t stable—they isomerize to aldehydes. Even so, some early preparations used acetaldehyde, the aldehyde that corresponds to vinyl alcohol. In a 1958 patent to Celanese (New York City), inventor Arthur W. Schnizer reported the reaction of acetaldehyde and acetic anhydride with a benzenesulfonic acid catalyst to produce vinyl acetate with 80–90% conversion.
That same year, Shelby P. Sharp and Alfred Steitz, Jr., at Pan American Petroleum (then a subsidiary of Standard Oil of Indiana) used the same reactants to form ethylidene diacetate, which was converted to vinyl acetate and acetic acid using a sulfuric acid catalyst. Previously, in 1935, Erich Rabald at C. F. Böhringer & Söhne (Mannheim–Waldhof, Germany) synthesized vinyl acetate from acetylene and acetic acid with a mercury catalyst, a process that also proceeded through ethylidene diacetate.
Almost all vinyl acetate now is produced via the vapor-phase reaction of ethylene and acetic acid over a noble-metal catalyst, usually palladium. The reaction is typically carried out at 175–200 ºC and 5–9 bar pressure. In the past 20 years, BP Chemicals, Celanese, Praxair, and Eastman Chemical have tuned this process to improve efficiency and decrease costs.
Current global vinyl acetate monomer demand is ≈6000 kt/year. Almost half is used to manufacture poly(vinyl acetate) homopolymer; the remainder goes into vinyl acetate copolymers and poly(vinyl alcohol) and its copolymers. The current VAM bulk price ranges from US$100 to $125, depending primarily on the country or region.