What molecule am I?
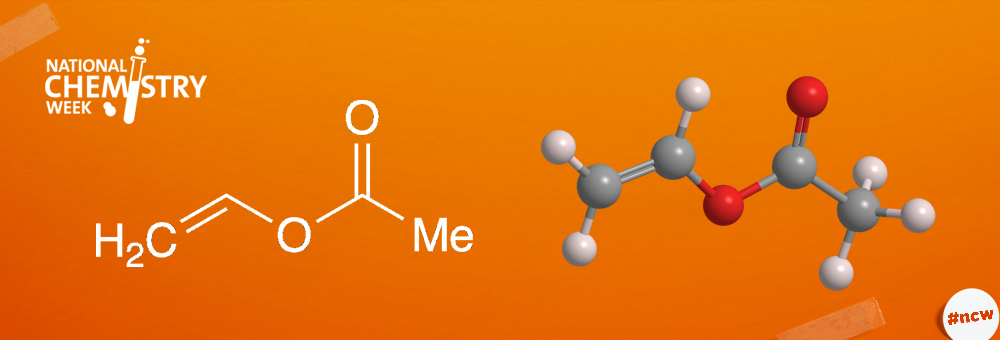
Vinyl acetate is an important industrial monomer that is used to make homopolymers and copolymers with a wide variety of applications. It is a volatile, flammable liquid that, like many esters, has a pleasant, fruity aroma.
Esters are usually synthesized from an alcohol and an acid; but in the case of vinyl acetate, the starting alcohol would be vinyl alcohol, which is not stable. To learn how vinyl acetate is made, see “Vinyl acetate synthesis and current production”.
The homopolymer poly(vinyl acetate)1 (PVA or PVAc) is an important polymer used in adhesives, textile sizing, and even chewing gum. In 1912, German chemist Fritz Klatte discovered PVA when he observed that vinyl acetate easily reacts with itself. Klatte’s PVA formulations were dense solids; but over the years, researchers developed methods for producing the polymer in other forms.
One of the most common forms of PVA is made by aqueous free-radical emulsion polymerization. Water, a surfactant, and an initiator (e.g., sodium persulfate) are mixed and heated. Vinyl acetate monomer (frequently called VAM in the industry) is added slowly, and the system is heated until the reaction is complete.2 The product is a milklike latex emulsion.
PVA latices are widely used in adhesives. One of the most familiar is Elmer’s Glue. PVA adhesives are used in packaging applications such as shipping boxes and bags, food containers, envelopes, tapes, and as binders for paper, plastics, and foils.
In addition to making homopolymers, vinyl acetate is combined with other monomers such as ethylene and acrylates to produce a variety of copolymers. Ethylene–vinyl acetate copolymers (EVAs) can be made in bulk or by emulsion polymerization. Emulsion EVAs have many uses, including adhesives that are similar to vinyl acetate homopolymers. Bulk polymer applications range from large-volume industrial uses to the handy glue sticks you may have at home or in school.
Vinyl acetate–acrylate emulsion copolymers (“acrylics”) are used primarily in paints and other coatings. They have largely replaced oil-based coatings for economic and environmental reasons.
This year’s National Chemistry Week’s theme, “Sticking with Chemistry”, pays homage to the numerous adhesive polymers prepared from vinyl acetate and other monomers. Be sure to check it out at www.acs.org/ncw.
1. Often incorrectly expressed as polyvinyl acetate.
2. In some cases, all of the PVA is added at the beginning of the process.
Vinyl acetate hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Flammable liquids, category 2 | H225—Highly flammable liquid and vapor | |
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Carcinogenicity, category 2 | H351—Suspected of causing cancer | |
| Hazardous to the aquatic environment, acute hazard, category 3 | H402—Harmful to aquatic life | |
* Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
Vinyl acetate fast facts
| CAS Reg. No. | 108-05-4 |
| SciFinder nomenclature | Acetic acid ethenyl ester |
| Empirical formula | C4H6O2 |
| Molar mass | 86.09 g/mol |
| Appearance | Colorless liquid |
| Boiling Point | 72.7 ºC |
| Water solubility | 20 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

