FOR IMMEDIATE RELEASE
ACS News Service Weekly PressPac: March 24, 2021
Creating patterns spontaneously in synthetic materials
“Spontaneous Patterning during Frontal Polymerization”
ACS Central Science
Nature produces a startling array of patterned materials, from the sensitive ridges on a person’s fingertip to a cheetah’s camouflaging spots. Although nature’s patterns arise spontaneously during development, creating patterns on synthetic materials is more laborious. Now, researchers reporting in ACS Central Science have found an easy way to make patterned materials having complex microstructures with variations in mechanical, thermal and optical properties –– without the need for masks, molds or printers.
In animals, patterns form before birth in response to genetically controlled signals that often vary in concentration along a gradient. In contrast, traditional manufacturing approaches for creating patterns and structures involve multiple steps, such as layer-by-layer assembly, lithography, molding or casting. Jeffrey Moore, Nancy Sottos, Philippe Geubelle and colleagues wanted to develop a spontaneous patterning method more akin to the biological process.
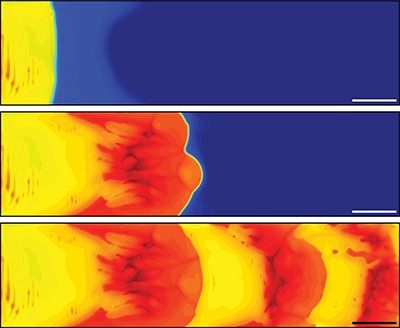
The team placed a solution of dicyclopentadiene monomer in a channel and then heated one end of the channel. The heat activated a ruthenium catalyst, which caused the monomer to polymerize, generating more heat that traveled down the channel. This continued to happen until all available monomer in the region was consumed. Eventually, heat traveled farther down the channel to a location where fresh monomer was present, and the process began again. With this technique, the team produced resins with regular ridges, and they controlled the height and spacing of the ridges by adjusting the initial temperature of the solution. By using a different monomer or adding other compounds to the solution, the researchers created materials with patterns of color and stiffness. The new method could someday be used to create a variety of new “smart” materials, from tire or shoe treads to electronics and biomaterials, the researchers say.
The authors acknowledge funding from the Air Force Office of Scientific Research, the Arnold & Mabel Beckman Foundation, the National Science Foundation and the Illinois Space Grant Consortium.
###
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
To automatically receive press releases from the American Chemical Society, contact newsroom@acs.org.
Note: ACS does not conduct research, but publishes and publicizes peer-reviewed scientific studies.
Media Contact
ACS Newsroom
newsroom@acs.org
View the image