Gas Chromatography-Mass Spectrometry
Dedicated at the H Hotel in Midland, Michigan, on June 8, 2019
In Star Trek, Mr. Spock’s hand-held tricorder can instantly tell what something is made of. We don’t have tricorders yet, but we’re getting close. Portable devices just a little too big to hold in one hand are used today to analyze samples at crime scenes, fires, and other places where time is of the essence.
The technology had its start 60 years ago in Midland, Michigan, with the pairing of two powerful analytical techniques — gas chromatography (GC) and mass spectrometry (MS). By coupling the ability of GC to separate a chemical mixture with the ability of MS to identify its components, the new, combined technique proved revolutionary. GC-MS is now routinely used for speedy analysis in forensics, environmental monitoring, drug testing of athletes, and other applications.
Contents

The origin of MS dates to the early 20th century, when Sir Joseph John “J. J.” Thomson of the University of Cambridge was studying the structure and behavior of atoms and molecules. Building on his and others’ previous research, Thomson in 1907 developed a device that created an electric arc in a container holding a small amount of a gas. The electrical discharge stripped electrons from the gas molecules, creating a variety of positively charged ions with a range of masses.
In the presence of an electric field, the ions could be accelerated and manipulated. When pushed through a magnetic field, the stream of ions bent and separated like light through a prism, Thomson discovered. The ions then struck a fluorescent screen or photographic plate at locations dictated by their mass-to-charge ratios, creating bright streaks where they landed. The resulting patterns were different for different materials, so Thomson could identify pure materials by their unique patterns.
Given this background, some historians credit Thomson as the inventor of MS. Most others look to his assistant, Francis W. Aston, who made multiple improvements and won a Nobel Prize in Chemistry in 1922 for the development of the first workable mass spectrograph.
By the mid-20th century, more-advanced mass spectrometers became commercially available. For each sample analyzed, the ions yielded a chart or “mass spectrum” from which the original molecule’s structure could be inferred.
If analyzed under identical conditions, any given compound always produces the same family of ions, creating a unique mass spectrum for each compound. When two or more compounds are present, the mass spectrum is a combination of the spectrum of each component. The result may be so messy it can’t be used to identify the components, meaning MS works well for pure materials, but not so well for mixtures.
“GC-MS is the synergistic combination of two powerful microanalytical techniques. The gas chromatograph separates the components of a mixture in time, and the mass spectrometer provides information that aids in the structural identification of each component.”—Gas Chromatography and Mass Spectrometry: A Practical Guide
The first widely noticed introduction of GC was made in 1951-52 by Anthony T. James and Archer J. P. Martin of the National Institute for Medical Research, in London. Commercial instruments soon followed. The technique built on earlier chromatography research by multiple scientists, including work that earned Martin and Richard L. M. Synge the 1952 Nobel Prize in Chemistry.
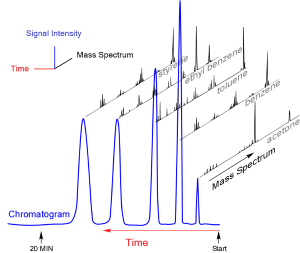
GC relies on the differing affinities of vapor components for surfaces. In a gas chromatograph, a mixture is first vaporized and picked up by an inert gas. This carrier gas is then pushed into a tube or “column” that in early days was packed with small, solid particles. Due to their different chemical properties some compounds interact with the solid surfaces more strongly than others and are slowed in their race through the column. At the end of the column is a specialized detector that produces a signal as each compound exits the column, with the signal intensity corresponding roughly to the relative amount of each component. Plotting the signal on graph paper (or in later years, on a computer screen) gives a peak for each component in the mix. The pattern of peaks, or “chromatogram,” is reproducible for any given sample, assuming it’s run through the column in the same way.
Many GC columns separate compounds approximately by boiling point. Low-boiling substances move faster and have lower retention times than higher-boiling substances. However, boiling points aren’t unique, so different chemicals can have the same retention time. That means chromatographic retention time alone isn’t enough to unambiguously identify a component in a mixture.
“GC-MS is indispensable in the fields of environmental science, forensics, health care, medical and biological research, health and safety, the flavor and fragrances industry, food safety, packaging, and many others.”—Gas Chromatography and Mass Spectrometry: A Practical Guide
In 1950, Fred McLafferty and Roland Gohlke, two Dow Co. researchers, dramatically enhanced the analytical power of GC by coupling it with MS. Adding MS allowed each component exiting the gas chromatograph to be analyzed separately.
Taken together, the mass spectra and the chromatographic peaks allowed unambiguous identification of each component. For an unknown mixture, the mass spectrum for each peak can narrow the possible identity of each component. Known standards can then confirm the identifications if both retention time and mass spectra match.
In coupling GC with MS, Gohlke and McLafferty overcame many issues. Chromatography columns weren’t commercially available, so they had to make their own. GC operates under pressure, whereas MS operates in vacuum. They had to devise a valving arrangement that would leak only a little of the total material coming from the gas chromatograph, without altering retention times. They also had to rapidly capture the fleeting mass spectrum for each compound: Lab computers didn’t exist at the time, so they photographed each spectrum as it briefly appeared on an oscilloscope.
After making a gas chromatograph and valve they thought would work, the researchers met with William C. Wiley and Daniel B. Harrington at Bendix Aviation Corp in Southfield, Michigan. There, McLafferty and Gohlke coupled their gas chromatograph with a very fast mass spectrometer that Wiley and his Bendix colleagues had developed. In short order they produced spectra of acetone, benzene, carbon tetrachloride, and toluene from a mixture of these compounds.
After this first successful demonstration of a paired GC-MS instrument in the winter of 1955-56, McLafferty and Gohlke convinced Dow to buy a Bendix mass spectrometer. Gohlke continued the GC-MS experiments at Dow’s spectroscopy lab with numerous colleagues. He and McLafferty first presented their results at the American Chemical Society’s April 1956 national meeting. Gohlke published the first journal article about their GC-MS work in Analytical Chemistry in 1959.
Around this same time, Joseph C. Holmes and Francis A. Morrell of Philip Morris Inc. also coupled GC and MS using a slower spectrometer made by Consolidated Engineering Corp., an approach the Dow scientists had rejected. Holmes and Morrell initially announced their findings at an American Society for Testing and Materials MS committee meeting in Cincinnati in May 1956. They wrote up their findings in a 1957 paper in Applied Spectroscopy.
Holmes and Morrell are credited by some for the development of GC-MS due to the independent but near-simultaneous demonstration. None of these four scientists patented the technology, leaving other researchers and companies free to adapt and improve on the method.
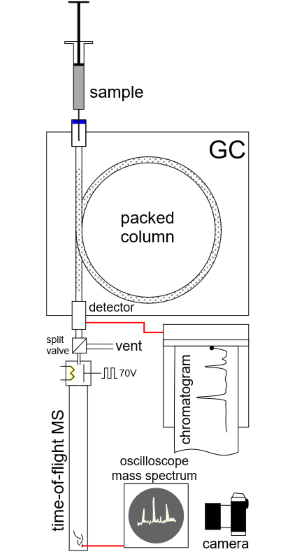
Mass spectrometers work on several different principles. The Bendix spectrometer used by Gohlke and McLafferty was a “time-of-flight” instrument that produced a spectrum based on the time it took ions to traverse a long tube. Bendix began marketing a GC-MS device in 1959, but the first commercial success was LKB Instruments Inc.’s Model 9000, which debuted in 1965. The LKB instrument used a magnet to disperse the ions just like Thomson did years earlier. Other companies followed suit, including Finnigan Instruments, Perkin Elmer, and Hewlett Packard, now Agilent.
Several other advances paved the way for GC-MS to go mainstream. The instruments became smaller and less expensive. With developments in computing power, libraries of mass spectra could be compiled and computers could identify chromatographic peaks.
GC-MS is an essential technology in modern analytical chemistry labs. Applications include development of new pharmaceuticals and analysis of their purity, detection of chemical warfare agents and explosives, screening of athletes’ urine for banned performance-enhancing substances, and analyzing soil samples on Mars. Portable units can now be carried in one arm for on-site analysis, bringing us closer than ever to Star Trek’s vision.
Landmark dedication
The American Chemical Society (ACS) honored The Dow Chemical Company’s innovation in combining gas chromatography and mass spectrometry with a National Historic Chemical Landmark (NHCL) in a ceremony at the H Hotel in Midland, Michigan, on June 8, 2019. The commemorative plaque reads:
In 1955-56, Dow Chemical scientists Fred McLafferty and Roland Gohlke first demonstrated the combination of gas chromatography (GC) and mass spectrometry (MS) to identify individual substances in a mixture. This was the first coupling of a separation technology with a spectrometry technique to provide rapid characterization of chemical components. GC-MS remains one of the most powerful, flexible, and widely used tools for analyzing chemical mixtures in drug screening, forensic, environmental, and trace analysis, as well as other applications.
Acknowledgements
Written by Mark Jones.
The author wishes to thank contributors to and reviewers of this booklet, all of whom helped improve its content, especially members of the ACS NHCL Subcommittee.
The nomination for this Landmark designation was prepared by the Midland Section of the ACS and The Dow Chemical Co.



