By Tracy P. Hamilton
We place batteries inside remote controls, toys (like the ones that light up or make sounds), wireless keyboards and mouses, wall clocks, and smoke detectors. Let’s take a look inside a single-use alkaline battery you might have at home.
What is a battery?
A battery is a storage device for energy. It stores chemical energy and converts it into electrical energy whenever you need it.
Parts of a battery
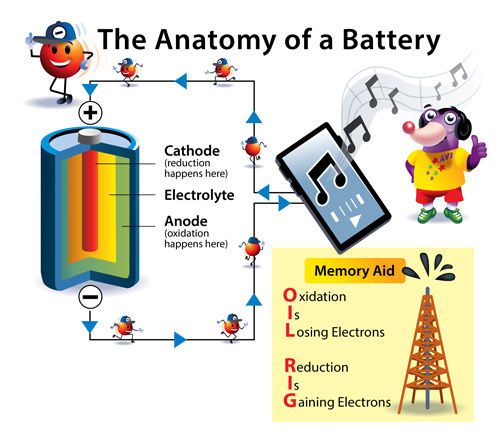
Look closely at the cylinder-shaped battery in the picture. It has two ends: one has a part that sticks out on its top. Next to it, you can see a little plus (+) sign. This is the positive end of the battery, or cathode. The completely flat end of the battery has a minus (−) sign next to it. This is the negative end of a battery, or anode. Depending on the battery type, there is also a liquid, solid, or paste/gel, called an electrolyte. The electrolyte separates the cathode and the anode.
Why do batteries “die”?
A battery works when the original chemicals inside it are still new and unused. When electricity starts flowing, these chemicals react with each other to become different chemicals. Once the original chemicals are all used up, the battery is dead. If you could reverse the reaction or add more of the original chemicals, you may be able to keep the reaction going.
A chemical reaction is a bit like building a little house with Legos. Once you have used up all your Lego pieces, the (re)action stops. If you want to build something new, you have two choices. You could choose to take the house apart and reuse the Legos, which is kind of how rechargeable batteries work. Or you could decide to buy more Legos, which is kind of like replacing dead batteries with new ones. Just like you would reuse your Legos to make something else, be sure to recycle your dead batteries. See Recycle That Battery! to find out how.
How does a battery work?
Everything around us is made of the smallest, basic building blocks called atoms. They make up everything from the chair you sit on, to your favorite book, to your own body! Atoms are extremely small. Even the dot over one “i” on this page is made of millions and millions of atoms.
Atoms have electrons, which are extremely tiny, negatively-charged particles. Batteries work by making these electrons move from one part of the battery to another. Batteries are made up of two parts. One part, the anode, “holds on” to its electrons very loosely. The other part is the cathode, and it has a strong pull on the electrons and holds them tightly.
Electricity is generated when electrons move from the anode (– end) to the cathode (+ end). The electrons don’t start moving until you pop the battery into a device and turn it on. Now the electrons can move from the anode to the cathode through your device. When electricity is flowing, the cathode gains the same number of electrons that the anode loses. This happens through two different types of chemical reactions. The reaction when the cathode gains electrons is called reduction. The reaction when the anode loses electrons is called oxidation.