Frost! As the seasons change, from winter to spring and summer to fall, farmers around the world struggle to protect their crops from frost damage.
In the spring, vulnerable buds that die in a late frost can mean no fall crops, and an early frost in the fall can mean a fully grown crop dies on the vine.
Frost occurs when water vapor in the air deposits on a surface when the outside temperature drops below 0 °C (32 °F). Because no liquid form of water is present, this is an example of deposition—a phase change directly from a gas to a solid without going through the liquid phase. Snowflakes are made this way too, but where they form on dust particles, frost grows on solid surfaces such as plants, trees, windows, and streetlights.
At the cellular level plants are predominately water. When plant cells freeze, they burst. This is because water expands when it freezes. Plants can withstand a few hours at temperatures below —0.6 °C (31°F), but a few minutes at –2 °C (28 °F) can result in plant death.
So, while frost is what we observe as an indication of dropping temperatures, it’s actually the cold temperatures that farmers have to fight. Farmers use two common methods to keep their orchards warm: spraying their crops with water to form a mixture of water and ice around the buds or fruit, or using mobile gas heaters to do the same to the frost as it forms.
It might seem odd to use ice to keep plants from freezing, but the goal is to keep the temperature at or above freezing. It’s a matter of energy and what happens during phase changes.
At the molecular level, the flow of energy is complicated. Heat is released when water freezes or fuels burn, but releasing energy may first require an initial input of energy. What we observe is the net result of all the associated molecular- or atomic-level shenanigans.
Using Ice to Protect Against Frost Damage
In 1920, a young researcher in Washington State, Daniel James “DJ” Crowley, suggested that the cranberry farmers in the area turn overhead sprinklers on their cranberry harvest before an early frost. The farmers were skeptical, but they did as he suggested. And it worked. What did DJ Crowley know that prompted him to make this suggestion?
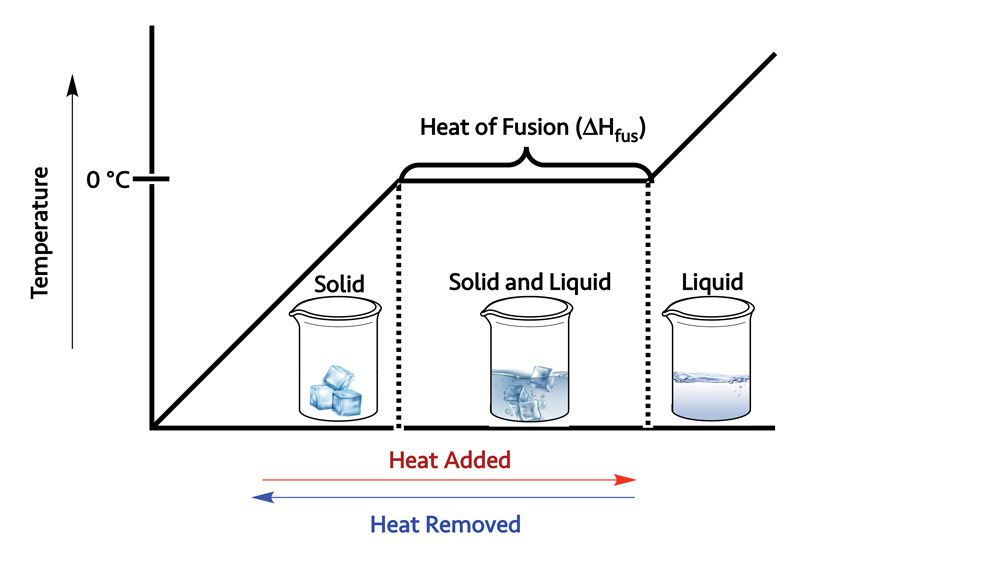
As unusual as it might sound, water, or rather a mixture of water and ice, can protect crops from dropping temperatures. When water and ice exist at the same time the temperature remains at 0 °C (32 °F) until the freezing process is complete, no matter how far the environmental temperature dips.
What is the connection between phase changes and energy?
Phase changes are driven by intermolecular forces of attraction between molecules. Like interpersonal relationships between you and your friends, intermolecular forces happen between one water molecule and its neighbors.
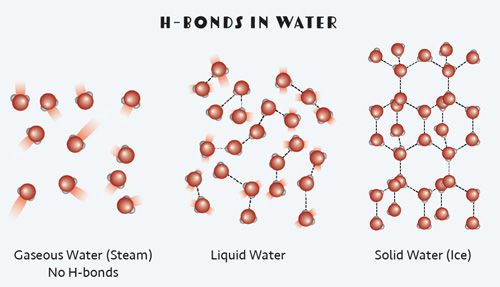
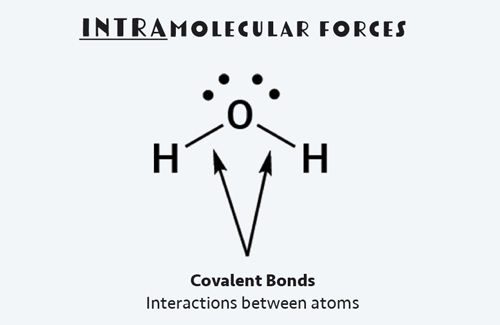
In water, these intermolecular forces are called hydrogen bonds, or H-bonds for short. And while they are called bonds, they are much weaker than intramolecular interactions, which occur between the individual water molecule’s oxygen and hydrogen atoms.
Remember, water has a dipole, that is, the more electronegative oxygen atom in the water molecule pulls the electrons towards itself.
This attraction of electrons toward the oxygen atom creates a partially negative charge on the oxygen. And, because the electrons are pulled away from the hydrogens, they become partially positively charged.
An H-bond is a very specific kind of intermolecular force that occurs between a molecule that contains hydrogen atoms bonded to highly electronegative atoms like oxygen. By convention, H-bonds are shown as dotted lines between the partial negative oxygen side of the water molecule to the partial positive hydrogen side of a different water molecule.
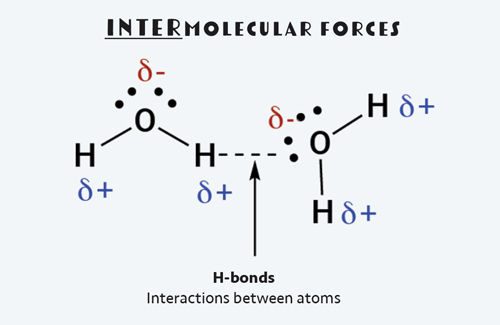
During a phase change from solid water (ice) to liquid water, some of the H-bonds between water molecules are broken. This takes energy from the environment. Conversely when water solidifies, energy is given off as additional H-bonds form between water molecules.
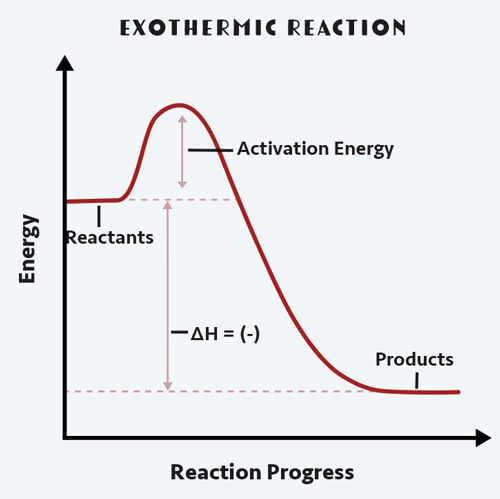
When energy is released into the environment, we call this exothermic. Heat is exiting the system: the prefix exo- is Greek for outside. We perceive this as a hot reaction: think campfire.
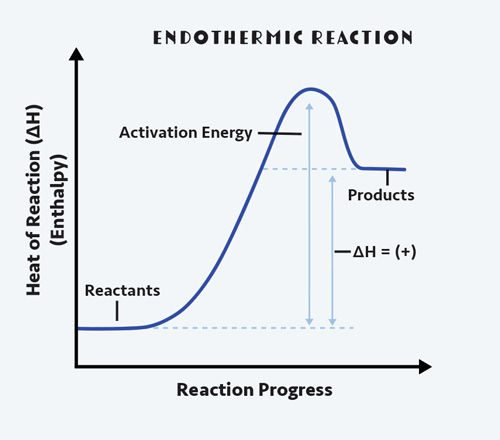
When energy is absorbed from the environment, we call this endothermic. Heat is being taken in: the prefix endo- is Greek for inside. We perceive this as a cold reaction: think ice cube melting in your hand. To break the hydrogen bonding interactions between water molecules in the solid phase, the ice pulls energy from your hand, making your hand feel cold.
During a phase change, the temperature of the system remains the same. The temperature won’t change until all of one phase has been converted to the other.
When two phases are present at the same time, energy is being absorbed and released. The energy required to melt ice or solidify water is 334 Joules per gram (J/g). This quantity is called the heat of fusion. The same amount of energy is released when water turns to ice, as is absorbed when ice turns to water. It just depends on whether we’re breaking or making H-bonds.
It is water’s heat of fusion and the associated temperature plateau that keeps the buds or fruit from freezing. As long as the ice that forms on the plants is kept wet, the temperature of the fruit inside will remain at or above 0 °C (32 °F). Also, as the ice freezes, the heat that is released as H-bonds form between water molecules, helps keep the encased fruit warm.
DJ Crowley’s knowledge of phase changes helped saved the cranberry crops in the 1920s, and his technolo-gy is still used today.
Using Combustion Reactions to Protect Against Frost
It might seem strange to think about heating an orchard using a gas heater. But high-powered heaters with jet-like blowers can be pulled behind a tractor to do just that.
Another option are to use upright heaters like those you might have seen on restaurant patios to extend the outside dining season. Along that same idea, some farmers use carefully placed barrels throughout a vineyard or orchard and light small wood fires.
All of these heating options are based on the same principle: Burn a carbon-based fuel to warm the air and melt the frost just enough to keep it wet because mixtures of solid and liquid water stay at 0 °C (32 °F).
Combustion reactions are common. We use them to heat our homes, cook our dinners, and power our cars. Unlike phase changes, the chemical composition of our fuel changes when it's burned. This means intramolecular forces—bonds between atoms—are broken and new ones are formed.

So, does the heat come from the breaking of the bonds or the forming of the bonds?
Let’s examine a common reaction, the combustion of methane (CH4), which occurs every time you light a Bun-sen burner or a gas stove in your home. When methane reacts with oxygen, it is converted to carbon dioxide and water.
CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g)
Overall, we know that this reaction is exothermic, because Bunsen burners are hot! But all chemical reactions have both an endothermic and an exothermic aspect to break bonds and rearrange them. Energy is always required to break intermolecular attractions or bonds between atoms.
First, energy must be absorbed by the methane and oxygen molecules. One way to visualize this is to think of two magnets. If they are stuck together, it will require energy to pull them apart.
Then to form the products—CO2 and H2O—new bonds must be formed between the carbon and the oxygens in carbon dioxide (CO2) and the hydrogens and the oxygen of water (H2O). When bonds form, energy is released. Thinking about the magnets again, if the magnets get close enough that they are attracted to each other, they may click together with an audible snap. The snap is energy being released in the form of sound waves. For atoms, energy in the form of heat is released when a bond forms.
The difference between the energy needed to break apart the bonds (pull the mag-nets apart) and the heat released to make new bonds (sound energy released by the magnets attracting each other) is called the enthalpy of reaction or ΔH (delta-H) when the reaction is carried out at constant pressure.
When a mole of water molecules form from hydrogen and oxygen, the heat released is 286 kilojoules (kJ). And carbon dioxide, because of its double bonds, releases even more energy. When a mole of CO2 forms from carbon and oxygen, 394 kJ of energy is released. A joule of energy is roughly equivalent to dropping a pear from the height of one meter. Imagine what 394,000 pears dropping from waist height might sound like!
When methane combusts, more energy is released when the products form than when the methane (CH4) and oxygen (O2) molecules are broken up. This makes the overall reaction exothermic.
In an exothermic reaction, the products at the end of the reaction have lower energy than the reactants. This difference in heat energy between the reactants and the products can then be used to save the harvest.
Luckily, heat doesn’t have to be applied to the whole orchard all at once. Remember, our goal is to have both ice and water present at the same time to keep the temperature around the fruit at 0 °C (32 °F). So, the farmers only need to warm their orchards enough to melt some of the frost, keeping both ice and water present at the same time.
Bonding and Energy
While bonding can be used to keep things warm, other chemical reactions can be very cool too—literally!
A very cool reaction involves mixing solid barium hydroxide octahydrate
(Ba(OH)2·8H2O) and ammonium thiocyanate (NH4SCN) and then resting it on a wet piece of filter paper on a piece of wood. The liquid slurry that forms gets so cold the water in the filter paper freezes to the wood and the beaker!
Ba(OH)2·8H2O(s) + 2 NH4SCN(s) → Ba(SCN)2(s) + 10 H2O(l) + 2 NH3(g)
When a reaction gets cold to the touch, it means the reactants are taking in more energy to break bonds than the products are releasing to form bonds. This means the reaction is endothermic.
Types of Frost
Frost happens when water vapor in the air deposits on a surface. Ice occurs when water liquid freezes.

Window Frost
Window frost forms when a pane of glass is exposed to below-freezing temperatures on the outside and warm air on the inside. The frost forms on the warm side of the glass and is an example of deposition—gas forming a solid without going through the liquid phase.

Hoar Frost
Hoar frost forms when water vapor freezes and the grains become long hair-like particles on a surface. Another example of deposition, the water particles form from the water in the air.

Frost Flowers
This rare phenomenon happens when water is pushed out of the pores of trees. It is not actually a frost, despite the name, because it is formed by freezing water.

Rime Frost
Rime frost forms when water vapor, in the form of fog close to the freezing point of water, comes into contact with surfaces that are below 0 °C (32 °F). Wind causes the fingers of rime frost to make long plumes of ice crystals.
REFERENCES
Janovich, A. Cranberries. Washington State Magazine, Winter 2021: https://magazine.wsu.edu/2021/11/08/cranberries/ [accessed Sept 2023].
The Endothermic Reaction Between Barium Hydroxide and Ammonium Thiocyanate. Science Skool. https://www.youtube.com/watch?v=PijjjHjjsck&t=45s [accessed Sept 2023].
Exothermic and Endothermic Reactions. American Chemical Society, Science Lesson Video: https://highschoolenergy.acs.org/how-can-energy-change/exothermic-endothermic.html [accessed Sept 2023].
Allain, R. We Need to Talk About the Energy in Chemical Bonds. Wired, Dec 7, 2015: https://www.wired.com/2015/12/we-need-to-talk-about-the-energy-in-chemical-bonds/ [accessed Sept 2023].
Baird, C. S. When Does the Breaking of Chemical Bonds Release Energy? Science Questions with Surprising Answers, June 27, 2013: https://www.wtamu.edu/~cbaird/sq/2013/06/27/when-does-the-breaking-of-chemical-bonds-release-energy/ [accessed Sept 2023].