An ancient chemical process enabled Earth to become a lush place teeming with life. Now researchers are replicating this process in an attempt to slow global warming.
Every plant, animal, and person owes their life to one sequence of chemical reactions: photosynthesis. The process, which converts water and carbon dioxide into food using sunlight, first evolved in cyanobacteria more than 2 billion years ago.
That’s right. Plants weren’t the first organisms to develop photosynthesis, though they are better known for it. Cyanobacteria are the ones that originally filled the atmosphere with photosynthesis’s gaseous by-product, oxygen (O2), which set the stage for more diverse life on Earth.
As beneficiaries of photosynthesis, humans depend on plants in a sort of carbon seesaw. Plants take in CO2 and release O2. They store that carbon as sugar. Hanging vines, grass, and trees all grow by pulling carbon atoms out of the air. We do the reverse, taking in O2 and releasing CO2. Finally, everything we eat completes the handoff: Human eats plant (or the animal who already did), human exhales, plant stores carbon, and the cycle continues.
This seesaw is part of the much broader carbon cycle that has affected the radiation balance of our planet. Cutting down huge swaths of forests and the burning of carbon-based fossil fuels causes the levels of CO2, a major greenhouse gas, to rise. And plants on Earth along with other natural parts of the carbon cycle can’t restore the balance on their own.
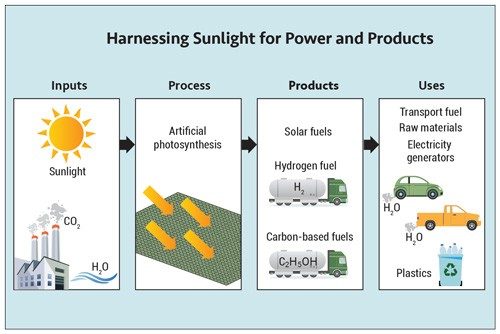
But what if we could copy what plants do to grab some of that excess CO2 to make fuels sustainably, instead of relying so heavily on fossilized carbon?
“Artificial photosynthesis is a really attractive approach,” says Jillian Dempsey, a chemist at the University of North Carolina, Chapel Hill. “You’re able to store the energy of the sun in the bonds of [molecules].”
At a large enough scale, such sun-powered processes could give us enough energy-storing molecules to make fuels out of thin air and water like plants and cyanobacteria do.
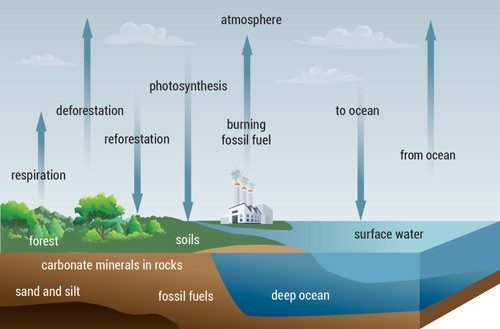
The Carbon Cycle
Carbon atoms go back and forth between the atmosphere and rocks, soil, plants, and the ocean via chemical processes; some processes are very slow, and others happen quickly. This constant exchange is called the carbon cycle. Human activities have disrupted the natural balance of this cycle by using once-buried fossil fuels, which would otherwise have kept a large amount of carbon beneath layers of the Earth.
Plants Make it Look Easy
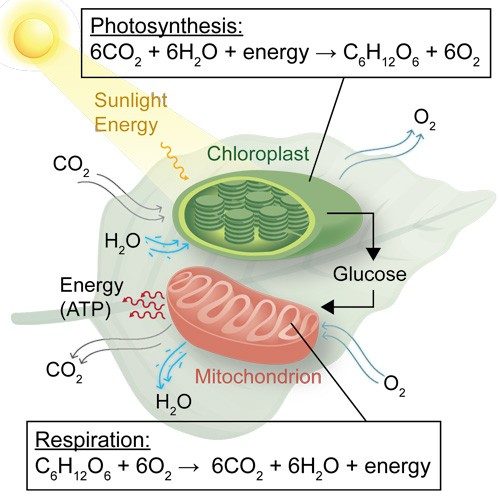
While it’s tempting to write photosynthesis off as a simple reaction—CO2 and water comes in, a leaf makes its food, and O2 heads out—the chemistry occurring is surprisingly complex. It’s a dance of protons, electrons, and biological machinery that had millions of years to evolve.
That machinery severs the strong bonds in H2O and CO2 to build more complex molecules, such as glucose (C6H12O6).
What the reaction equations don’t show is what spurs the reactions: the catalysts, which are substances that speed up specific chemical reactions. With the right catalysts, researchers have succeeded in building small devices that not only mimic natural photosynthesis, but also beat it.
Photosynthesis 2.0
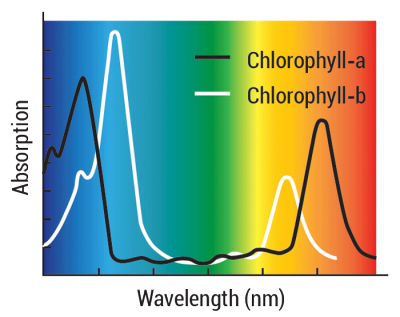
Let’s take a look at the role catalysis plays in photosynthesis. First, plants absorb sunlight in their chloroplasts, which are organelles found in plant and algae cells. Inside the chloroplasts are chlorophyll pigments that absorb mostly red and blue, but not green wavelengths of light (thus their color). This absorbed energy powers the plant’s chemical reactions.
Artificial photosynthesis needs its own version of chlorophyll. To find substances to act like chlorophyll in an artificial leaf, scientists are testing natural pigments, synthetic dyes, or other materials that absorb visible light.
In plants, once light is absorbed, a complicated chain of biological catalysts called enzymes carry out reactions in a precise order to create fuels such as glucose. In an artificial leaf, a similar thing must happen—except instead of glucose, the leaf should produce a fuel, such as ethanol, that our energy-driven technologies can use. Alternatively, some artificial leaf technologies don’t require CO2 but rather take in water to produce hydrogen fuel (H2).
Pigment Power!
To make their own food, green plants contain chlorophyll pigments that absorb energy to drive chemical reactions. Pigments absorb light with levels of energy that are just right to excite their electrons. The type and amount of a molecule’s bonds influence what those absorption wavelengths actually are. Chlorophyll-a and chlorophyll-b are important pigments that enable photosynthesis in green plants. They absorb light in the violet-blue and orange-red parts of the electromagnetic spectrum.
The Simplest Fuel
Hydrogen, when consumed in a fuel cell, produces no carbon emissions—its only emission is water. Today, most H2 is produced from methane (CH4) in a process that emits CO2.
If scientists could figure out how to make H2 using sunlight in an affordable way, hydrogen fuels would be more sustainable.
To do this with an artificial leaf, it would have to carry out the oxidation and reduction reactions required to make H2 from H2O, or “water splitting.” Like photosynthesis, the reactions seem straightforward on paper.
2H2O → O2 + 4H+ + 4e-
4H+ + 4e- → 2H2
But in practice, water splitting is an electrochemical process that relies on electricity to power the oxidation and reduction reactions.
“If I just put a cup of water out in the sunshine, it’s not going to spontaneously turn into hydrogen and oxygen,” says Dempsey, who designs catalysts for solar-fuel production.
H2O doesn’t easily part with its electrons and protons, or hydrogen ions (H+). Electrolysis needs a steady flow of external electrical energy, which can be supplied by a solar-powered artificial leaf. A current helps separate the oxygen atom from the hydrogen ions by pulling electrons away from the water molecule (oxidation). Those electrons flow from one part of the electrochemical cell, known as the anode, to another, known as the cathode. There, H+ ions pair up with electrons (reduction) to form H2 gas, which forms bubbles that can then be captured for use.
Catalysts, such as iron oxide and platinum, speed up the forming and breaking of chemical bonds.
“Catalysis gives us access to seemingly impossible chemical transformations,” Dempsey says.
Making Carbon Fuels
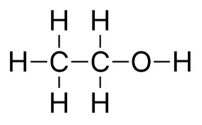
Ethanol, (C2H5OH), typically makes up 10% of the gas you pump into most cars. Some flex-fuel cars can handle up to 85% C2H5OH.
Today, most ethanol in the United States comes from fermenting crops, which rely on a lot of land, water, and energy from fossil fuels to grow. This is where artificial-leaf researchers come in. They are engineering catalysts to produce ethanol and butanol
(C4H9OH) in a more sustainable way. Butanol has similar uses to ethanol.
“To build carbohydrates or ethanol or butanol,” says Dempsey, “we need to carefully choreograph how the protons and the electrons are being reassembled.”
Turning a couple of CO2 molecules into C2H5OH requires shuffling around 12 protons and 12 electrons.
2CO2 + 12H+ + 12e- → C2H5OH + 3H2O
Scientists design new catalysts for artificial photosynthesis to make the process as efficient and sustainable as possible.
“We have gotten to the point where scientists have done enough preliminary studies to know that what we are proposing is achievable,” Dempsey says.
Butanol
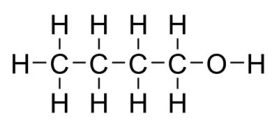
Ethanol
Natural Inspiration
What’s extraordinary is that these chemical choreographies work. Light, CO2, and water go in; fuel comes out. And in some ways, chemists have designed catalysts to make these fuels more efficiently than plants produce sugars.
“It turns out that natural photosynthesis isn’t actually that efficient of a process,” Dempsey says. Only about 1% of the solar energy that hits a plant turns into fuel energy. The efficiency of artificial leaf technologies can exceed 20%.
This doesn’t mean artificial photosynthesis copies natural photosynthesis in all aspects though. Plants are less efficient, but they make more complex fuels with greater precision than artificial devices. “Some of these systems that are showing promise make a whole slew of stuff—12 different products mixed together,” Dempsey says.
A major current challenge in catalyst research involves designing materials that speed up specific reactions and make only the products we want.
Making an Artificial Leaf
So, what would an artificial leaf device actually look like?
“It basically looks like this sandwich structure—the catalyst layers are sandwiching the photo-absorber,” says Peter Agbo, a staff scientist at Lawrence Berkeley National Lab who works with the Joint Center for Artificial Photosynthesis.
Existing devices are also small. A hydrogen device with 12.6% efficiency that Agbo recently built was less than one inch across. For artificial photo-synthesis to become practical, it needs to produce fuels at a large scale to compete with the world’s existing energy supply of relatively inexpensive and abundant fossil fuels.
Scaling up artificial photosynthesis is still far off, but it’s moving along.
“We’ve identified the fundamental scientific challenges,” Dempsey says. “We’re learning how to put the different pieces together.”
What might ultimately get the technology across the finish line is another turn back to nature—but this time, instead of just copying it, scientists want to use it. Engineers from the University of California at Berkeley, for example, recently combined nanoparticles with living nonphotosynthetic bacteria.
Their microbe of choice, Moorella thermoacetica, naturally reduces CO2 to make a small amount of acetic acid (CH3COOH). When the researchers fed the bacteria tiny clusters of gold atoms and exposed them to sunlight, the gold clusters were able to pull electrons from the amino acid cysteine and send them to the microbe’s enzymes. The electrons interacted with the bug’s enzymes, which spurred the bacteria to make much more acetic acid from CO2 than they normally would have. The acetic acid can then be used to make fuels and other valuable chemicals.
It will take a lot of time and money before artificial photosynthesis can compete with fossil fuels. But the needed investment won’t come close to the societal cost of climate change. A recent survey of more than 2,000 economists projected the economic damages from climate change will reach $1.7 trillion per year by 2025 and roughly $30 trillion per year by 2075.
Artificial photosynthesis could inch us back toward a better balance on the planet’s carbon seesaw.

REFERENCES
Hisatomi, T.; Kubota, J.; Domen, K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chemical Society Reviews, 43(22), pp 7520–7535; Jan 13, 2014: https://pubs.rsc.org/en/content/articlelanding/2014/cs/c3cs60378d [accessed Aug 2021].
Kistler, T. A.; Danilovic, N.; Agbo, P. A Monolithic Photoelectrochemical Device Evolving Hydrogen in Pure Water. Journal of the Electrochemical Society, 166(13), H656; June 9, 2019: https://iopscience.iop.org/article/10.1149/2.1151913jes [accessed Aug 2021].
Yang, P. Liquid Sunlight: The Evolution of Photosynthetic Biohybrids. NanoLetters, American Chemical Society, 21(13), pp 5453−5456; July 2, 2021: https://pubs.acs.org/doi/10.1021/acs.nanolett.1c02172 [accessed Aug 2021].