Downloads:
Suppose you wake up feeling ill on a weekday. What do you do? Do you go to school and hope you feel better soon, or stay home? If you go to school, you risk getting others sick if you have a viral infection. But staying home might mean missing out on an important test or team practice, and ultimately your symptoms could go away in just a couple of hours.
Making these decisions for just one person can be difficult. Imagine how hard it is to make the call for millions of people—especially when the scientific research that could inform a decision isn’t yet robust enough.
This is what public health officials often have to do when weighing the benefits of new (or old) products or activities against any potential health or environmental effects and consumers’ right to decide for themselves the amount of risk they’re willing to take.
Taking precautions
Sometimes the need to ban a product for a particular use is clear. For example, the anti-nausea drug thalidomide was used in the late 1950s and early ‘60s to treat morning sickness. But about 10,000 infants of women who were taking the drug were born with severe birth defects. The need for banning the drug during a woman’s pregnancy was indisputable.
In other cases, the question of how best to regulate a product is not as easily addressed. The public health debate over vaping is an example of how complicated an issue can be.
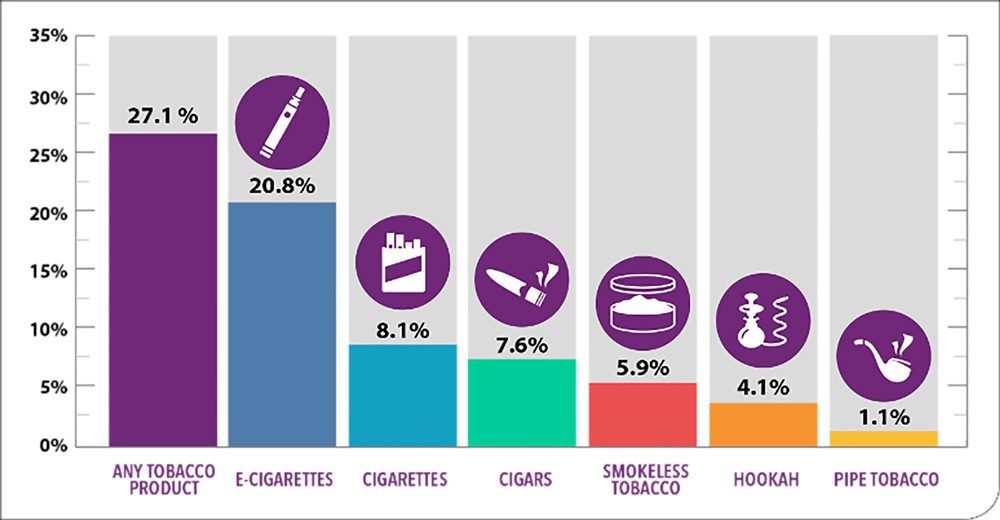
E-cigarettes containing nicotine were first introduced in 2004 and marketed by companies as a healthier alternative to smoking traditional cigarettes, which cause hundreds of thousands of deaths every year.
What’s clear about this issue is that nicotine is highly addictive and can cause a variety of negative health effects. To protect teens from exposure to nicotine, the United States banned sales of e-cigarettes and e-liquids to minors. But multiple red flags point to the need for additional action. Teen use has dramatically increased, despite the prohibition of selling to minors. E-cigarette companies haven’t provided the U.S. Food and Drug Administration with evidence to support the claims that vaping is less W harmful than smoking. And a severe lung illness associated with e-cigarette devices emerged this summer.
Determining what to do about e-cigarettes would be simpler if we knew more about how vaping affects health. As of early November, investigators didn’t have enough information to pinpoint the specific cause—or causes—of the new vaping-related lung disease. Also, the potential long-term effects of vaping haven’t been identified. The products had hit the market without testing for any such effects.
Minimizing harm
What we know so far about e-cigarettes is enough for some local governments in the U.S. and national governments elsewhere to implement partial or total bans on vaping products, particularly flavored e-liquids. They’re applying a guideline often used in public health, called the precautionary principle. This principle states that when an activity raises a threat of harm to human health or the environment, precautionary measures should be taken, even if cause and effect aren’t fully understood. Better safe than sorry, as the saying goes.
Newspapers have reported an immediate outcry from retailers and their customers who say that banning e-cigarettes will cause people to go back to traditional cigarettes.
So, what additional precautions can be put in place to protect the public from the health risks of both vaping and regular cigarettes? This question is open for discussion.