Ames Laboratory and Uranium Production in World War II
Dedicated at the Ames Laboratory in Ames, Iowa, on May 17, 2022.
The year was 1942 — deep into World War II — and almost overnight, uranium had become a very important element to top-secret groups. Scientists employed in massive programs in Germany and the U.S. believed the metal could be used to create a powerful new weapon. The war could potentially be won by the first nation to solve the puzzles of large-scale production and purification of uranium, and separation of its isotopes.
This Landmark honors the advances that overcame the first two of those three challenges. Those achievements resulted from a heroic effort by a dedicated group of scientists and technicians at a college in the middle of Iowa. Their little-known story is an integral and essential part of the Manhattan Project, which was a decisive factor in ending World War II.
Contents
View larger image

View larger image
Discovery of fission
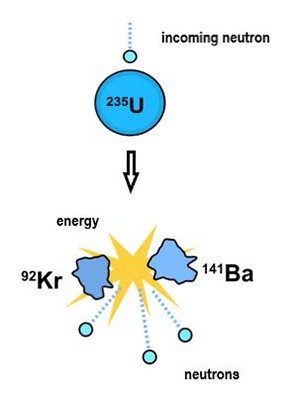
What led to this unprecedented situation? In early 1939, German chemists Otto Hahn and Fritz Strassmann, and Austrian physicists Lise Meitner and Otto Frisch, reported on experiments in which uranium (U) was bombarded with neutrons, subatomic particles with no charge. The results showed that uranium could undergo nuclear “fission,” a previously unknown nuclear reaction in which an atom absorbs a neutron and then splits into two lighter atoms, while emitting energy.
Each incoming neutron enables the release of two or more neutrons. Under the right conditions, the neutrons produced would bombard other uranium atoms, which in turn would release neutrons to strike even more atoms, expanding the fission process exponentially. Researchers believed this could lead to a chain reaction that would release massive amounts of energy almost instantaneously. It would be the key to a super weapon.
Late in 1939, Albert Einstein and other eminent scientists informed U.S. President Franklin D. Roosevelt that nuclear fission could possibly be used in the construction of extremely powerful uranium-based atomic bombs. They also warned the president that Nazi Germany was carrying out research on uranium.
This helped motivate the U.S. government to launch what came to be known as the Manhattan Project, the massive top-secret effort to undertake atomic research and develop a bomb before Germany could do so.
The need for scientific action was urgent. Concern increased when scientists found that it was not possible to produce a chain reaction of sufficient strength for an atomic weapon with natural uranium, which consists of a mix of isotopes. Only enriched uranium, in which the isotope U-235 greatly predominates, can sustain the chain reaction to that degree.
Isotopes of elements differ in their numbers of neutrons. All neutral atoms of a particular element contain the same number of protons and electrons, but the number of neutrons varies, leading to isotopes of different atomic weights. For example, naturally occurring chlorine contains a mixture of about 75% Cl-35 and 25% Cl-37 atoms; the Cl-37 isotope contains two more neutrons than Cl-35.
Naturally occurring uranium is a mixture of U-238 (99.274%) and a much smaller amount of U-235 (0.72%). Thus, because the concentration of U-235 in natural uranium is so low, the challenge of producing a sustained chain reaction was now increased by orders of magnitude. To get enough U-235 for a weapon, researchers would need to develop efficient methods to process, purify, and cast huge amounts of natural uranium, and to subsequently separate its isotopes. Purification was necessary because impurities remaining from the original uranium ore or preliminary refining steps could absorb the neutrons that were key to starting and sustaining a chain reaction. Casting was desirable in part because some forms of uranium — particularly powder — had a tendency to burst into flame.
Nuclear vs. chemical energy
Traditional energy sources rely on chemical energy, produced by making and breaking the chemical bonds connecting atoms via chemical reactions. The atoms remain unchanged by these reactions. In contrast, nuclear energy comes from destruction of the atoms themselves. Both types of reactions can release energy, but the production of nuclear energy is far more efficient on a weight basis. A pound of nuclear fuel releases the same energy as millions of pounds of a conventional fuel.
Iowa State joins the Manhattan Project
To oversee the crucial chemical and metallurgical components of this undertaking, project leaders decided to approach Frank Spedding, a physical chemistry professor at Iowa State College (now Iowa State University), located in the town of Ames.
Early in 1942, Spedding agreed to head the chemistry division of the Manhattan Project’s Metallurgical Laboratory. The lab was studying nuclear chain reactions of uranium and its conversion into plutonium, another fissionable element being considered as the basis for bombs. But there was very little space or equipment for the desperately needed chemical and metallurgical work on uranium at the University of Chicago, where the Metallurgical Laboratory was based. Since Iowa State had the necessary metallograph (a microscope for studying the structure of metals), a metallurgical furnace, and sufficient laboratory space, it was agreed that the work would begin at Ames. Thereafter Spedding spent half of each week working there on the “Ames Project.”
That he could oversee research both at Iowa State and in Chicago was made physically possible by an arrangement with the station manager at the Ames train depot, who reserved a sleeper car berth for Spedding’s commute back and forth. From a scientific standpoint, this worked because his Iowa State colleague in the project, chemistry professor Harley Wilhelm, was not just a master metallurgist, but also a natural leader. Thus, the work effort was seamless in Ames and greatly benefitted from the creativity and focus of both scientists. (Wilhelm’s vital role was recognized by naming one of Ames Laboratory’s first two buildings for him. It is adjacent to Spedding Hall.)
Still, the Ames Project team that they led would face a daunting challenge: how to produce ultrapure uranium from source materials in the huge quantities needed for an atomic weapon.

Operated under contract by Iowa State University with funding from the U.S. Department of Energy, [Ames Laboratory] was established in the 1940s following its development of highly pure materials for atomic research. In fact, Ames Lab scientists developed the most efficient process for producing pure uranium metal for the Manhattan Project during World War II.”
— DOE science feature, April 5, 2011
Purifying uranium
For at least 200 years, uranium metal had been sporadically produced in small amounts.
This was done with reduction techniques that were commonly used to free metals from their oxide ores, with varying degrees of success. In 1939, two companies in the U.S. were producing uranium commercially. Metal Hydride Inc. used a combination of heat and chemical reactions to produce uranium metal from UO2, while Westinghouse Electric and Manufacturing Co. opted for an electrolysis method that deposited uranium on a metal cathode.
In late 1941 and early 1942, the Manhattan Project asked these companies to consider expanding their production to tons of metal. This turned out to be impractical. Nevertheless, the two firms were able to supply sufficient amounts of uranium so the Ames team could begin studying the best way to melt and cast the metal for use in bomb development.
By June 1942, researchers at the lab had shown that the best approach was to melt the metal in a process known as vacuum-induction heating, and then cast it in graphite crucibles, which transferred almost no carbon impurities to the cooling uranium. For the rest of the year, uranium melting and casting at Ames was done by placing the metal on a graphite grill in the bottom of a graphite crucible seated in a graphite mold. This unit was induction heated under vacuum in a large silica tube fitted with a vacuum head. The resulting liquid uranium metal flowed from the crucible through the grill into the heated mold, a process called drip casting.
During this same time, the search continued for a better way to convert uranium raw materials to uranium metal on a large scale and with high purity. In early studies, the reduction was done directly by reacting uranium oxide and carbon, but the resulting metal always contained carbon impurities that made it unacceptably brittle. Trials with other reductants such as hydrogen, magnesium, and aluminum also failed.
In the summer of 1942, another path opened up. Small amounts of UF4 became available to the Metallurgical Laboratory, and Spedding brought some to Ames. In an initial experiment there, researchers combined UF4 with calcium and heated the mixture under vacuum. When the apparatus was subsequently reopened, they found that heat produced by the reaction had driven the reduction of UF4 to metallic uranium to completion. Pure uranium metal had agglomerated into a large chunk that was easily separated from calcium fluoride (CaF2) at a yield of more than 60%. The scientists had succeeded in developing an efficient process to purify uranium:
UF4 + 2 Ca ➞ U + 2 CaF2 + heat
In late September, some of the Ames uranium was cast into a single ingot weighing 11 pounds and measuring 2 inches in diameter and 5 inches in length. This was taken to Chicago, where Spedding, in a triumphant moment, placed it on the desk of Metallurgical Project Director (and Nobel laureate) Arthur Compton, who had set up the Metallurgical Lab. As it was then almost inconceivable to have that much pure uranium, Compton disparagingly stated, “I’ll wager there is a big shrinkage cavity inside.” Compton was fortunate that no one accepted the bet, because cutting the ingot down the middle revealed not the slightest indication of an internal void.
On December 2, 1942 — using tons of uranium supplied by Ames, Metal Hydrides, and Westinghouse — the Metallurgical Lab launched the world’s first controlled, self-sustaining, nuclear chain reaction. The experiment, in the reactor dubbed Chicago Pile-1, demonstrated that military use of nuclear energy might be feasible.
By March 1943, the Ames team shifted to reduction of UF4 with magnesium at a higher temperature. Although this method was more complicated, magnesium was readily available and was less expensive, more pure, and more efficient to use than calcium. The process was scaled up, and Ames scientists also developed a method to recover uranium from scrap metal. Ultimately, the site produced more than two million pounds of pure uranium metal for use in the Manhattan Project’s atomic weapon development. The Ames methods reduced production costs by as much as twenty-fold compared with others under development at the time. Industry gradually took over uranium production using the Ames processes, and by the end of the war in 1945, Iowa State ended most of its uranium operations.
When uranium is dug out of the ground, it has less than 1% of the usable isotope. It also has other impurities that would get in the way of doing nuclear fission.”
— Ames Laboratory Director Adam Schwartz, Iowa Public Radio’s River to River program, January 24, 2022
Other chemistry milestones at Ames
Other important chemistry advances have taken place at Ames Laboratory. For instance, in the 1970s, Ames scientists Velmer Fassel and Sam Houk developed the use of inductively coupled plasma to prepare samples for analysis with mass spectrometry. The technique is used for applications such as environmental monitoring, geochemical analysis, metallurgy, pharmaceutical analysis, and clinical research.
In 1996, Ames Laboratory patented multiplexed capillary electrophoresis, which can quickly and inexpensively analyze the chemical composition of multiple samples at once. The method is used widely in pharmaceutical, medical, and genetic research fields and was vital to sequencing the human genome. The technique was developed by Edward Yeung and colleagues at Ames Lab and Iowa State.
In 1997, the Laboratory patented a new type of solder, which is used to join metal components. Traditional solder was typically made of toxic lead, which could leach into the environment from land-filled electronics waste. To eliminate much of the toxic metal from electronic devices, lab researchers including Iver Anderson created a solder made of silver, copper, and tin.
Founding of Ames Laboratory
After the war, the federal government continued funding the research at Iowa State. In 1947, as a result of the Ames Project’s success, Ames Laboratory was formally established on the campus as a national laboratory of the recently launched Atomic Energy Commission. It is now part of the Department of Energy.
The Ames Project and its successor, the Ames Laboratory, have provided a unique educational experience for many graduate students and postdoctoral fellows at Iowa State. The lab continues to serve as a model for developing major research relationships with the federal government. Today, it is a multifaceted scientific enterprise with a strong focus on materials science.
It is not an exaggeration to state that without the achievements of the Ames Laboratory on the Iowa State campus in the years 1942-45, World War II could have been considerably lengthened, with significant further loss of human life. The key role played by the laboratory in the Manhattan Project ultimately enabled both military and peaceful uses of atomic energy.

A key figure
At first glance, it is puzzling that Frank Spedding, a young Iowa State professor who had never been involved in uranium research, would be asked to direct some of the nation’s best-known atomic chemists for the Manhattan Project. But he was uniquely qualified, thanks to his varied training and his expertise in the chemistry of rare earth metals, which are chemically similar to the actinide series of elements that include uranium.
Spedding, who was born in 1902, held a B.S. Spedding, circa 1944 in chemical engineering, an M.S. in analytical chemistry of metal alloys, and a Ph.D. in chemistry. He did his doctoral work at the University of California, Berkeley with Gilbert N. Lewis. After working as an instructor and research fellow at Berkeley, he was awarded a Guggenheim Fellowship, during which he spent some time at the Cavendish Laboratory at Cambridge University in England and with the physicist Niels Bohr in Copenhagen.
Upon returning to the U.S., Spedding took a temporary two-year assistant professorship at Cornell University, through 1937. When this position ended, Spedding learned that Iowa State had an opening for a physical chemist. He drove to Ames, Iowa, where Winfred Coover, chair of the chemistry department, offered him a job as an assistant professor on the spot. However, Spedding — who was tired of short-term assignments — would not agree to anything less than a tenured associate professorship, which was quickly approved by the Board of Regents.
Spedding joined what came to be known as the Manhattan Project when he launched the Ames Project in 1942. After the end of World War II in 1945, he was appointed director of Iowa State’s newly founded Institute for Atomic Research. This was succeeded by the Ames Laboratory, which was formally established at Iowa State in 1947 by the Atomic Energy Commission. Spedding served as the laboratory’s first director and held that post until 1968. He died in 1984.

Landmark dedication and acknowledgments
Landmark dedication
The American Chemical Society (ACS) honored Ames Laboratory’s production of uranium during World War II with a National Historic Chemical Landmark (NHCL) designation in a ceremony at the laboratory in Ames, Iowa, on May 17, 2022. The commemorative plaque reads:
Nuclear fission — the process of splitting atoms — was discovered in 1939. Scientists predicted the process could be harnessed to produce energy and powerful weapons. The U.S. government launched the Manhattan Project to develop this technology, including the high purity uranium required to sustain the necessary fission chain reactions. In 1942, Iowa State chemistry professor Frank Spedding established and directed a chemical research and development program, known as the Ames Project. Led by chemist Harley Wilhelm, it devised a process for producing uranium pure enough to enable chain reactions. The Ames Project produced 1,000 tons of purified uranium for the Manhattan Project, advancing national security and the development of nuclear power. Following this success, Ames Laboratory was established at Iowa State in 1947.
Acknowledgments
Written by Tom Barton, Iowa State University.
The author wishes to thank contributors to and reviewers of this booklet, all of whom helped improve its content, especially members of the ACS NHCL Subcommittee.
The nomination for this Landmark designation was prepared by Ames Laboratory, Iowa State University, and the Ames Section of the ACS.

Booklet PDF
Additonal resources
Further reading
- The Ames Project video (Ames National Laboratory)
- Manhattan Project Roots (Ames Lab)
- Ames Lab overview (Ames Lab)
- About Ames National Laboratory (Ames Lab)
- Fission and Fusion: What is the Difference? (Department of Energy)
- The Ames Project: Administering classified research as a part of the Manhattan Project at Iowa State College, 1942-1945 (Iowa State University dissertation)
- Nuclear Fission, 1938-1942 (American Institute of Physics)
- Discovery of Nuclear Fission (American Physical Society)
- Ames Lab Director talks all things Ames Lab on Iowa Public Radio (Ames Lab)
- Einstein's Letter to President Roosevelt - 1939 (atomicarchive.com)

