Joseph Priestley and the Discovery of Oxygen
Dedicated August 1, 1994, at the Joseph Priestley House in Northumberland, Pennsylvania, USA, and August 7, 2000, at Bowood House in Wiltshire, UK.
Priestley House Commemorative Booklet (PDF)
Bowood House Commemorative Booklet (PDF)
En español: Joseph Priestley y el descubrimiento del oxígeno
Landmark Lesson Plan: Joseph Priestley, Discoverer of Oxygen
When Joseph Priestley discovered oxygen in 1774, he answered age-old questions of why and how things burn. An Englishman by birth, Priestley was deeply involved in politics and religion, as well as science. When his vocal support for the American and French revolutions made remaining in his homeland dangerous, Priestley left England in 1794 and continued his work in America until his death.
Contents
About Joseph Priestley
Some 2,500 years ago, the ancient Greeks identified air — along with earth, fire and water — as one of the four elemental components of creation. That notion may seem charmingly primitive now. But it made excellent sense at the time, and there was so little reason to dispute it that the idea persisted until the late 18th century. It might have endured even longer had it not been for a free-thinking English chemist and maverick theologian named Joseph Priestley.
Priestley (1733-1804) was hugely productive in research and widely notorious in philosophy. He invented carbonated water and the rubber eraser, identified a dozen key chemical compounds, and wrote an important early paper about electricity. His unorthodox religious writings and his support for the American and French revolutions so enraged his countrymen that he was forced to flee England in 1794. He settled in Pennsylvania, where he continued his research until his death.
The world recalls Priestley best as the man who discovered oxygen, the active ingredient in our planet's atmosphere. In the process, he helped dethrone an idea that dominated science for 23 uninterrupted centuries: Few concepts "have laid firmer hold upon the mind," he wrote, than that air "is a simple elementary substance, indestructible and unalterable."
In a series of experiments culminating in 1774, Priestley found that "air is not an elementary substance, but a composition," or mixture, of gases. Among them was the colorless and highly reactive gas he called "dephlogisticated air," to which the great French chemist Antoine Lavoisier would soon give the name "oxygen."
It is hard to overstate the importance of Priestley's revelation. Scientists now recognize 92 naturally occurring elements-including nitrogen and oxygen, the main components of air. They comprise 78 and 21 percent of the atmosphere, respectively.
Understanding the Composition of Air
In the mid-18th century, the concept of an element was still evolving. Researchers had distinguished no more than two dozen or so elements, depending on who was doing the counting. It wasn't clear how air fit into that system. Nobody knew what it was, and researchers kept finding that it could be converted into such a variety of forms that they routinely spoke of different "airs."
The principal method for altering the nature of air, early chemists learned, was to heat or burn some compound in it. The second half of the 1700s witnessed an explosion of interest in such gases. The steam engine was in the process of transforming civilization, and scientists of all types were fascinated with combustion and the role of air in it.
British chemists were especially prolific. In 1754, Joseph Black identified what he called "fixed air" (now known to be carbon dioxide) because it could be returned, or fixed, into the sort of solids from which it was produced. In 1766, a wealthy eccentric named Henry Cavendish produced the highly flammable substance Lavoisier would name hydrogen, from the Greek words for "water maker."
Finally in 1772, Daniel Rutherford found that when he burned material in a bell jar, then absorbed all the "fixed" air by soaking it up with a substance called potash, a gas remained. Rutherford dubbed it "noxious air" because it asphyxiated mice placed in it. Today, we call it nitrogen.
But none of those revelations alone tells the whole story. The next major discovery would come from a man whose early life gave no indication that he would become one of the greatest experimental chemists in history.
Bubbling Beverages
In 1767, Priestley was offered a ministry in Leeds, Englane, located near a brewery. This abundant and convenient source of "fixed air” — what we now know as carbon dioxide — from fermentation sparked his lifetime investigation into the chemistry of gases. He found a way to produce artificially what occurred naturally in beer and champagne: water containing the effervescence of carbon dioxide. The method earned the Royal Society's coveted Copley Prize and was the precursor of the modern soft-drink industry.
Oxygen and Other Discoveries in England
Joseph Priestley was born in Yorkshire, the eldest son of a maker of wool cloth. His mother died after bearing six children in six years. Young Joseph was sent to live with his aunt, Sarah Priestley Keighley, until the age of 19. She often entertained Presbyterian clergy at her home, and Joseph gradually came to prefer their doctrines to the grimmer Calvinism of his father. Before long, he was encouraged to study for the ministry. And study, as it turned out, was something Joseph Priestley did very well.
Aside from what he learned in the local schools, he taught himself Latin, Greek, French, Italian, German and a smattering of Middle Eastern languages, along with mathematics and philosophy. This preparation would have been ideal for study at Oxford or Cambridge, but as a Dissenter (someone who was not a member of the Church of England) Priestley was barred from England's great universities. So he enrolled at Daventry Academy, a celebrated school for Dissenters, and was exempted from a year of classes because of his achievements.
After graduation, he supported himself, as he would for the rest of his life, by teaching, tutoring and preaching. His first full-time teaching position was at the Dissenting Academy in Warrington. (Although obviously brilliant, original, outspoken and, by one report, of "a gay and airy disposition," Priestley had an unpleasant voice and a sort of stammer. That he made a living through lectures and sermons is further evidence of his extraordinary nature.)
In 1762, he was ordained and married Mary Wilkinson, the daughter of a prominent iron-works owner. She was, he noted, "of an excellent understanding, much improved by reading, of great fortitude and strength of mind, and of a temper in the highest degree affectionate and generous; feeling strongly for others and little for herself."
Priestley traveled regularly to London, and became acquainted with numerous men of science and independent thought, including an ingenious American named Benjamin Franklin, who became a lifelong friend. Franklin encouraged Priestley in his research, one result of which was The History and Present State of Electricity. For that work, and his growing reputation as an experimenter, Priestley was made a Fellow of the Royal Society in 1766.
The History book was too tough for a popular audience, and Priestley determined to write a more accessible one. But he could find no one to create the necessary illustrations. So, in typical fashion, he taught himself perspective drawing. Along the way, he made many mistakes, and discovered that India rubber would erase lead pencil lines — a fact he mentioned in the preface.
By the age of 34, Priestley was a well-established and respected member of Britain's scientific community. He was still paying a price for his religious nonconformity, however. When the explorer Captain James Cook was preparing for his second voyage, Priestley was offered the position of science adviser. But the offer was rescinded under pressure from Anglican authorities who protested his theology, which was evolving into a strongly Unitarian position that denied the doctrine of the trinity.
In retrospect, the Cook affair may have been all for the best. In 1773, the Earl of Shelburne asked Priestley to serve as a sort of intellectual companion, tutor for the earl's offspring, and librarian for his estate, Bowood House. The position provided access to social and political circles Priestley could never have gained on his own, while leaving ample free time for the research that would earn him a permanent place in scientific history.
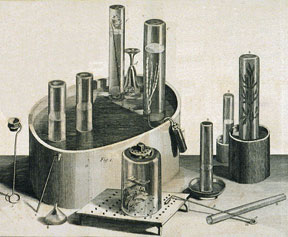
He systematically analyzed the properties of different "airs" using the favored apparatus of the day: an inverted container on a raised platform that could capture the gases produced by various experiments below it. The container could also be placed in a pool of water or mercury, effectively sealing it, and a gas tested to see if it would sustain a flame or support life.
In the course of these experiments, Priestley made an enormously important observation. A flame went out when placed in a jar in which a mouse would die due to lack of air. Putting a green plant in the jar and exposing it to sunlight would "refresh" the air, permitting a flame to burn and a mouse to breathe. Perhaps, Priestley wrote, "the injury which is continually done by such a large number of animals is, in part at least, repaired by the vegetable creation." Thus he observed that plants release oxygen into the air — the process known to us as photosynthesis.
On August 1, 1774, he conducted his most famous experiment. Using a 12-inch-wide glass "burning lens," he focused sunlight on a lump of reddish mercuric oxide in an inverted glass container placed in a pool of mercury. The gas emitted, he found, was "five or six times as good as common air." In succeeding tests, it caused a flame to burn intensely and kept a mouse alive about four times as long as a similar quantity of air.
Priestley called his discovery "dephlogisticated air" on the theory that it supported combustion so well because it had no phlogiston in it, and hence could absorb the maximum amount during burning. (The year before, Swedish apothecary Carl Wilhelm Scheele isolated the same gas and observed a similar reaction. Scheele called his material "fire air." But his findings were not published until 1777.)
Whatever the gas was called, its effects were remarkable. "The feeling of it in my lungs," Priestley wrote, "was not sensibly different from that of common air, but I fancied that my breast felt peculiarly light and easy for some time afterwards. Who can tell but that in time, this pure air may become a fashionable article in luxury. Hitherto only two mice and myself have had the privilege of breathing it."
Phlogiston and Fire
In the mid-18th century, the most pressing issue in chemistry and physics was to determine what exactly happens when something burns. The prevailing theory was that flammable materials contained a substance called “phlogiston” (from the Greek word for burn) that was released during combustion.
The theory held that when a candle burned, for example, phlogiston was transferred from it to the surrounding air. When the air became saturated with phlogiston and could contain no more, the flame went out. Breathing, too, was a way to remove phlogiston from a body. A typical test for the presence of phlogiston was to place a mouse in a container and measure how long it lived. When the air in the container could accept no more phlogiston, the mouse would die.
The 18th century scientist Antoine Lavoisier disproved the existence of phlogiston and helped to form the basis of modern chemistry using Joseph Priestley’s discovery of oxygen.
Religion and Emigration to America
As luck would have it, Lord Shelburne was setting off on a trip to the European continent and took Priestley along. In France, Priestley met Lavoisier and described his discovery. It turned out to be the clue Lavoisier needed to develop his theory of chemical reactions — the "revolution" in chemistry that would finally dispel the phlogiston theory. Burning substances, Lavoisier argued, did not give off phlogiston; they took on Priestley's gas, which Lavoisier called "oxygen" from the Greek word for acid-maker.
By then, however, Priestley had returned to England, where he escalated his support for the American Revolution and for highly unorthodox religious views. Those positions were a source of embarrassment for Lord Shelburne. Priestley left his service in 1780, moving to Birmingham and taking a position as head of a liberal congregation called New Meeting.
His new location brought him into contact with numerous luminaries including Erasmus Darwin, grandfather of Charles, the great architect of evolutionary theory. James Watt and Matthew Boulton — who were about to transform society with their steam engine — were there, as was Josiah Wedgwood, the famous potter, who supported Priestley's chemical experiments. Birmingham also boasted a distinguished scientific discussion group, the Lunar Society, which met on nights of a full moon so that the members could see their way home.
Priestley's encouragement of the French Revolution, together with his increasingly controversial theology and attacks on the doctrine of the trinity, eventually became too notorious for safety. In 1791, an alcohol-fueled mob of royalists burned the New Meeting house, and then Priestley's home. The scientist and his family barely escaped. They fled to London, but eventually it proved no safer. Priestley's sons could not find work and emigrated to Pennsylvania, where they hoped to found a center for free-thinking Englishmen.
Finally Joseph and Mary followed them, setting sail for America on April 8, 1794. Priestley turned down the offer of a teaching position at the University of Pennsylvania in Philadelphia, and instead built a house in the remote hamlet of Northumberland to be near his sons. The area was decidedly rustic.
There Priestley continued his research, isolating carbon monoxide (which he called "heavy inflammable air") and founding the Unitarian Church in the United States. For the most part, he led a quiet and reflective life — especially after his friend Thomas Jefferson was elected president in 1800.
During his final trip to Philadelphia, he told the Philosophical Society that "having been obliged to leave a country which has been long distinguished by discoveries in science, I think myself happy by my reception in another which is following its example, and which already affords a prospect of its arriving at equal eminence." His words proved prophetic. A colloquium held on the centennial of Priestley's discovery of oxygen led to the founding of the American Chemical Society —today the world's largest scientific society — in 1876.
On February 3, 1804, Priestley began an experiment, but found himself too weak to continue. He went to his bed in his library, never again to emerge. On February 6, he summoned one of his sons and an assistant. He dictated some changes in a manuscript. When he was satisfied with the revisions, he said "That is right. I have now done." Minutes later he died painlessly, ending what Jefferson called "one of the few lives precious to mankind."
Further Reading
- The Joseph Priestley House (Pennsylvania Historical & Museum Commission)
- Joseph Priestley (Chemical Heritage Foundation)
- En español: Joseph Priestley y el descubrimiento del oxígeno
Landmark Designation and Acknowledgments
Landmark Designation
The American Chemical Society dedicated the Joseph Priestley House a National Historic Chemical Landmark on August 1, 1994. The plaque commemorating the event reads:
Joseph Priestley (1733-1804) — Unitarian minister, teacher, author, natural philosopher, discoverer of oxygen, and friend of Benjamin Franklin and Thomas Jefferson — supervised the construction of this house and laboratory from 1794 to 1798, then lived and worked here until his death in 1804. His library of some 1,600 volumes and his chemical laboratory, where he first isolated carbon monoxide, were probably the best in the country at that time. As suggested by Edgar Fahs Smith in 1920, the Joseph Priestley House has become "a Mecca for all who would look back to the beginnings of chemical research" in America.
The American Chemical Society and the Royal Society of Chemistry dedicated the Discovery of Oxygen by Joseph Priestley an International Historic Chemical Landmark on August 7, 2000, in Wiltshire, UK. The plaque commemorating the event reads:
Joseph Priestley (1733-1804) — Unitarian minister, teacher, author, and natural philosopher — was the Earl of Shelburne's librarian and tutor to his sons. In this room, then a working laboratory, Priestley pursued his investigations of gases. On 1 August 1774 he discovered oxygen. Twenty years later he emigrated to America where he continued his research at his home and laboratory in Northumberland, Pennsylvania.
Acknowledgments:
Adapted for the internet from “The Joseph Priestley House,” produced by the National Historic Chemical Landmarks program of the American Chemical Society in 1994 and “Bowood House,” produced by the National Historic Chemical Landmarks program of the American Chemical Society and the Royal Society of Chemistry in 2000.
Learn more: About the Landmarks Program
Take action: Nominate a Landmark and Contact the NHCL Coordinator
Teach: Landmark Lesson Plans