Saul Hertz and the Medical Uses of Radioiodine
Dedicated at Massachusetts General Hospital, Boston, on Oct. 8, 2021.
On a spring day in Boston in 1941, a young doctor from Massachusetts General Hospital (MGH) met a woman for a drink. But this was no social call, and the setting was anything but relaxing. Dr. Saul Hertz was serving an atomic cocktail to Elizabeth D., a patient experiencing symptoms of an overactive thyroid. In the hopes of curing what ailed her, she bravely swallowed the concoction of synthetic radioactive iodine produced at Massachusetts Institute of Technology (MIT).
The occasion marked a marriage of practical medicine and particle physics that would help give birth to the field of nuclear medicine. Thanks to developments by Hertz and other researchers, radioiodine (RAI) and other radioactive pharmaceuticals are now routinely used in diagnostic imaging and treatment of disease, thereby improving or saving the lives of millions of people.
Contents
This budding relationship between medicine and physics began soon after Frédéric Joliot and Irène Joliot-Curie received the 1935 Nobel Prize in Chemistry in recognition of their creation of new radioactive isotopes. At a Harvard Medical School colloquium on Nov. 12, 1936, MIT President Karl Compton gave a lecture on “What Physics Can Do for Biology and Medicine” in which he mentioned the production of novel radioactive isotopes and potential applications of radioactive substances in medicine.
Hertz, director of the thyroid clinic at MGH, was in attendance with other physicians, including Dr. James Howard Means, MGH chief of medicine, who had established the clinic. Hertz was an endocrinologist and thyroid specialist. The lumpy, butterfly-shaped gland in the human neck had captured his imagination. He marveled at how it regulates metabolism, normally with pinpoint accuracy — until it doesn’t. He was passionate about his work fixing malfunctioning thyroids, a condition that happens in more women than men and causes problems including weight loss, fatigue, anxiety, or irritability.
Surgery to remove overactive thyroid tissue in patients with Graves’ disease was costly and could be dangerous, so Hertz and Means were looking for a nonsurgical alternative. Bombarding a patient with X-rays was also used to destroy overactive tissue, but it was problematic since the radiation damaged healthy tissue as well.
Previous scientific work had established that the thyroid absorbs iodine and needs it to function properly. Lack of iodine causes enlargements known as goiters. Hertz, listening to Compton’s lecture, suddenly made a connection. He asked Compton a pivotal question: “Could iodine be made radioactive artificially?”
Compton researched the answer and confirmed Hertz’s hunch a month later. Enrico Fermi had made iodine radioactive in 1934 and published his results in Nature. The iodine isotope, I-128, has a 25-minute half-life. That means half the radioactive iodine atoms in a sample of I-128 decay in that amount of time, releasing radiation in the process. The remaining I-128 atoms in the sample continue to decay by half every 25 minutes, until the radioactivity has dissipated.
A delighted Hertz figured the radioactive decay of I-128 could kill overactive thyroid tissue. But unlike X-rays, it would leave other healthy tissue unharmed, because iodine is selectively taken up by the thyroid. In a Dec. 23, 1936, letter to Compton he wrote that he hoped the radioactive iodine would “…be a useful method of therapy in cases of overactivity of the thyroid gland.”
Hertz and his research partner at MIT, a young physicist and accomplished pianist named Arthur Roberts, wasted no time in exploring the potential treatment. In 1937, backed by funding from Harvard, they injected lab rabbits with small amounts of I-128. Roberts had used a radium-beryllium neutron source to create the radioiodine at MIT’s Radioactivity Center, which was headed by Robley Evans.
The researchers took note of readings from a Geiger counter and calculated that almost all the iodine was taken up in the rabbits’ thyroids or flushed out in urine; the radioactive element didn’t accumulate in other organs. Even better, abnormal thyroid tissue absorbed more iodine than normal glands. These studies showed that radioactivity could be used to trace the movement of iodine through the body and to probe thyroid physiology. One day soon, Hertz predicted, RAI might also cure toxic thyroids, goiters — even cancer. But to use the treatment in human subjects, the researchers needed an iodine isotope with a longer half-life.
At the time, curing cancer with beta particles ejected by radioactive isotopes decaying into stable atoms may have seemed pie-in-the-sky to most physicians, but Hertz wasn’t alone in his forward thinking.

The fact that iodine is selectively taken up by the thyroid gland when injected into the body makes it possible to hope that iodine which is made radioactive ... will be a useful method of therapy in cases of overactivity of the thyroid gland." — Saul Hertz in a Dec. 23, 1936, letter to MIT President Karl Compton
Around the same time as Hertz’s encounter with Compton in 1936, a Yale University research physician named John Lawrence was heading across the country by train, riding third class because it was the only way he could bring his traveling companions (hundreds of stinky lab rodents). The young doctor had been invited out to the University of California (UC), Berkeley by his older brother, Ernest Lawrence. Just a few years before, Ernest had created one of the greatest inventions of 20th century physics: the atom-smashing cyclotron, which earned him a Nobel prize.
“My brother and I always thought there was a terrific opportunity in biology and medicine to bring in physics, chemistry, mathematics, all the basic sciences which were neglected in medical schools,” John Lawrence recalled in a 1979 interview. He began studying how radioactive phosphorus, created by the new cyclotron, might help cure his rats and mice of leukemia by concentrating in the bone marrow to kill leukemia cells. He would later test the treatment in leukemia patients and, in 1939, successfully used it to treat patients with polycythemia, a fatal clotting disease.
But in the meantime, Ernest tasked John with finding other medical researchers interested in using radioactivity. “We wanted to get [radioactive isotopes] out, both for therapy and for tracer research,” John said.
Unfortunately, it was a tough sell, and he wasn’t getting many takers. One of them was Dr. Joseph Hamilton, a medical school resident with an affinity for chemistry, who came from UC San Francisco wanting to study radioiodine as a tracer — a way to follow the metabolic path of a substance — in the human body.
There was one critical matter: time. The available iodine radioisotope, I-128, remained stable only long enough to study metabolism in rodents or rabbits. As Hertz and Roberts knew, it wasn’t practical for people, because the isotope decayed too rapidly to get good data in the human system.
In the spring of 1938, Hamilton searched out the one man he thought could come up with the exact radioisotope tracer he needed to study humans. He cornered chemist Glenn Seaborg on the UC Berkeley campus. Hamilton “was doing work on thyroid metabolism using iodine-128, which had a half-life of 25 minutes,” Seaborg told the Journal of Nuclear Medicine, “and he complained bitterly that this really cramped his style.” Seaborg asked Hamilton to be specific: What exactly did he need? Hamilton responded that a tracer with a half-life of about a week would be great.
Soon after, Hamilton got the news. Seaborg and his research partner, physicist John Livingood, were at the Berkeley cyclotron fabricating the perfect tracer tool. By bombarding tellurium with deuterons, they’d synthesized iodine-131. The new isotope had a half-life of eight days — almost exactly what the doctor ordered. They also produced other isotopes including I-130, which has a half-life of about 12 hours.
John Lawrence said Hamilton was particularly good at convincing patients to participate in the research trials; all they would have to do was drink a cocktail of radioactive I-131 produced by the Berkeley cyclotron. Hamilton did this tracer study of iodine metabolism in partnership with Mayo Soley, an MGH-trained internist from the thyroid clinic at the University of California Hospital, San Francisco. As with Hertz’s rabbits the year before, the team found that the human thyroid gland could take up radioiodine.
It is comforting to know that so many people are well now because of the scientific expertise of people like Dr. Hertz." — First Lady Barbara Bush, who was successfully treated for thyroid disease, in a May 22, 1989, letter to Hertz’s widow, Vitta Hertz
Back in Boston, the MIT-MGH team wanted their own source of long-lived radioiodine, so they explored the possibility of building a cyclotron at MIT. In those days, money for research came mainly from contributions by wealthy donors and private foundations, and scientists commonly had to make their own requests. In this case, the Markle Foundation in New York was persuaded to finance a brand new cyclotron.
By March 31, 1941, Hertz and Roberts were ready with radioiodine prepared by the Markle cyclotron. Most of the iodine was I-130, while a small amount was the longer-lived I-131.
They gave a cocktail of the radioiodine in water to Elizabeth D. — making her the first patient to be treated with radioiodine for thyroid disease. Roberts took measurements of uptake of the isotope with a Geiger counter positioned near Elizabeth’s neck.
“Radioactivity was a glorious and wonderful thing,” Evans said in a 1978 interview, noting that before World War II there was little fear of radioactivity in general. The patients received “a glass of water with radioactive iodine, and this was just great. The media loved it. The public loved it.”
Hertz continued giving the cocktail to patients. Out in California in fall 1941, Hamilton, Soley and John Lawrence started therapeutic studies as well. Both teams were seeing terrific results with goiters shrinking in many patients and almost no complications. Hertz reported at a 1942 conference that the radioactive iodine “goes directly into the enlarged thyroid gland and within fifteen minutes gives off enough radiation directly inside the thyroid gland to become a promising therapeutic agent.”
The concept of “theranostic” radioactive drugs that could be used to both diagnose and treat disease was looking to be a big success. Hertz’s 1936 hypothesis had been spot on.
In 1943, Hertz temporarily left his practice and his thyroid research and joined the war effort. He volunteered with the U.S. Navy Medical Corps, helping advance atomic medicine. Meanwhile, Evans and MGH’s Dr. Earle Chapman continued the work.
After the war, in 1946, the Boston and California teams presented their multi-year and multi-patient studies. The results were impressive, with the majority of patients completely cured of Graves’ disease.
Still, Hertz knew that synthesizing radioiodine in the cyclotron was tedious, expensive, and time consuming, and he feared it would be difficult to carry out wide-scale studies or treatments. Hertz lobbied for the military to release the radioactive iodine left over from making atomic weapons at Oak Ridge, Tennesee. He was successful. A promising new therapy to save lives was born from the creation of a weapon to do just the opposite.
Hertz continued to study the relationship between iodine radioactivity and the thyroid gland and pioneered targeted therapy for thyroid cancer treatment. His work, and the studies by the Lawrence brothers, Joseph Hamilton, and others, laid the foundation for the field of nuclear medicine. Their advances were followed by discoveries by other brilliant men and women who applied complicated concepts of physics, chemistry and medicine to build on the pre-war breakthroughs.
Radioiodine was used beginning in 1947 for probing the brain for tumors, in 1950 for imaging how blood pools in the heart, in 1955 for measuring cardiac output and imaging the liver, and in 1982 for treating malignant melanoma — and it’s still used today. Other radiopharmaceuticals continue to be developed for diagnostic imaging and therapy.
Millions of patients — including Seaborg’s mother, who was cured of hyperthyroidism thanks in part to her son’s development of iodine-131 — have enjoyed better health due to the discoveries of doctors and scientists who collaborated across disciplines.
Today, medical uses of [radioiodine] remain the gold standard of targeted precision oncology." — “Remembering Dr. Saul Hertz,” Congressional Record, May 11, 2021
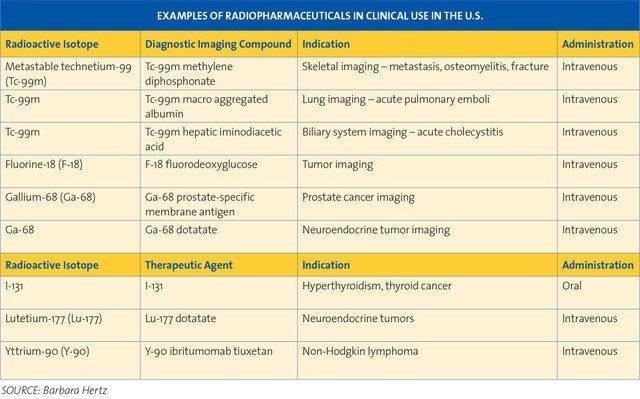
High-resolution table

Booklet PDF
Landmark dedication
The American Chemical Society (ACS) honored Saul Hertz and the Medical Uses of Radioiodine with a National Historic Chemical Landmark (NHCL) designation in a ceremony at Massachusetts General Hospital in Boston on Oct. 8, 2021. The commemorative plaque reads:
Shortly after scientists first produced new radioactive isotopes, Saul Hertz, M.D., chief of Massachusetts General Hospital’s Thyroid Clinic, realized it might be possible to make radioactive iodine to treat diseases of the thyroid — as this gland accumulates iodine. Hertz and Massachusetts Institute of Technology physicist Arthur Roberts, Ph.D., began experimenting with radioiodine in 1937 and administered the first treatment to a Massachusetts General patient on March 31, 1941. Hertz later used radioiodine to diagnose and treat cancer. These advances, and discoveries by other researchers, laid the foundation for nuclear medicine. Radioiodine and other radiopharmaceuticals are now routinely used in diagnostic imaging and treatment of disease, saving and improving the lives of millions of people.
Acknowledgements
Written by Victoria Bruce.
The author wishes to thank contributors to and reviewers of this booklet, all of whom helped to improve its content, especially members of the ACS NHCL Subcommittee.
The nomination for this Landmark was prepared by Barbara Hertz, the Northeastern Section of the ACS, and the ACS Division of Nuclear Chemistry and Technology.
Additional resources
Further reading
- Saul Hertz MD website
- “Celebrating Eighty Years of Radionuclide Therapy and the Work of Saul Hertz,” by F. H. Fahey and F. D. Grant, J. Appl. Clin. Med. Phys. 2021, 22 (1), 4–10
- “Saul Hertz, MD, and the Birth of Radionuclide Therapy,” by Frederic Fahey, Frederick Grant and James H. Thrall, EJNMMI Physics 2017, 4 (15)
- “John H. Lawrence: Nuclear Medicine Pioneer and Director of Donner Laboratory,” University of California, Berkeley, 1979, by Sally Smith Hughes
- “Donner Laboratory: Fifty Years of Nuclear Medicine Innovation,” by Karla Harby, J. Nucl. Med. 1988, 29 (4), 431-437
- “The Life and Contributions to the Periodic Table of Glenn T. Seaborg, the First Person to Have an Element Named after Him While He Was Still Alive,” by D. Seaborg, Pure Appl. Chem. 2019, 91 (12), 1929–1939
- Historical Timeline: Important Moments in the History of Nuclear Medicine, Society of Nuclear Medicine and Molecular Imaging
- “Radioactive Iodine in the Study of Thyroid Physiology. VII. The Use of Radioactive Iodine Therapy in Hyperthyroidism,” by Saul Hertz and Arthur Roberts, JAMA. 1946, 131(2), 81-86
En español: Saul Hertz y los usos del yodo en radiomedicina



