Birth of the Petrochemical Industry
A National Historic Chemical Landmark
Dedicated at Clendenin, West Virginia, on Sept. 10, 2021
Today’s enormous petrochemical industry is built on a technology that began modestly in the hills of West Virginia. In 1920, the Carbide and Carbon Chemicals Corporation was formed as a subsidiary of the Union Carbide and Carbon Company. It was established to develop a process to manufacture ethylene, which could then be converted into several industrially useful compounds. The move to ethylene was a revolutionary step because acetylene was the workhorse chemical of the day.
The new firm owned a small plant in Clendenin, West Virginia, that separated natural gasoline from raw natural gas. The gasoline, which at room temperature is a liquid, was sold as fuel.
But the remaining liquids derived from natural gas, consisting mostly of ethane and propane, found no ready markets, so the company installed processing units and a furnace to convert them into more valuable products. The furnace began operating in 1921 to produce ethylene.
At the time, the Union Carbide and Carbon Company could not have known that the advances achieved in the small town of Clendenin would lay the foundation for a multi-billion-dollar petrochemical industry that affects virtually every aspect of modern life.
Ethylene has become the largest-volume synthetic organic chemical in the world. Most ethylene is used to produce polyethylene. Now the world’s most common plastic, polyethylene is used in both rigid and flexible packaging, piping, electrical insulation and more.
Ethylene is also key in the production of many other chemicals, including polyvinyl chloride, polyethylene terephthalate, ethylene glycol, polystyrene and styrenic polymers, and vinyl acetate. It is the foundational molecule that supports the modern chemical industry.
Contents

Ethylene had been known to chemists since the 18th century. Typically made from ethanol and sulfuric acid, it was used to fuel gas lights and to produce some other chemicals. But as of 1920, the chemical industry wasn’t based on ethylene. Instead, it centered on acetylene, a gas used as a fuel for car headlights, welding and cutting, and as the feedstock for making several other chemicals.
However, ethylene offered many advantages over acetylene in terms of chemical synthesis, and it was also far safer to use. George Oliver Curme Jr. (1888-1976) provided the vision for the process to make ethylene, for recognizing the range of products that could be made from it, and for the marketing of these chemicals.
Yet Curme’s career actually began with a focus on acetylene. After earning a Ph.D. in chemistry at the University of Chicago and spending a year in Germany at the Kaiser Wilhelm Institute, in 1914 he became the Prest-O-Lite Illumination Fellow at Mellon Institute in Pittsburgh, with the goal of developing an alternative method for the synthesis of acetylene.
Prest-O-Lite made acetylene lamps for cars and bikes. In the early 1900s, the company was one of the largest consumers of calcium carbide, which produces acetylene when reacted with water. However, Union Carbide had a near monopoly on calcium carbide at that time, so Prest-O-Lite wanted to find an alternative production route for the acetylene to fuel its lamps.
Curme and his coworkers succeeded: By striking mineral oil with an electric arc, causing it to heat up and decompose, they produced acetylene as well as the by-product ethylene. Curme knew it would improve the method’s viability if he could find a profitable use for the ethylene, so he began investigating commercially practical processes to convert it into other chemicals. As it turned out, he found that it could be used to produce almost every chemical made from acetylene — and others too.
Then, in 1917, Union Carbide (which is now part of Dow Chemical Company) merged with Prest-O-Lite, the Linde Air Products Company, and the National Carbon Company to form the Union Carbide and Carbon Company. Curme continued his ethylene work at the newly formed company.

Dr. George Oliver Curme is recognized as ‘the father, grandfather, and great-grandfather of ethylene and her numerous progeny.’" — “George Oliver Curme, Jr.,” a memoir by Augustus B. Kinzel
Although the arc process was successful in producing acetylene and ethylene, it was still quite expensive because of the substantial amount of electric power required. Nor was the historic process of making ethylene a good alternative, because it required large amounts of dangerous sulfuric acid.
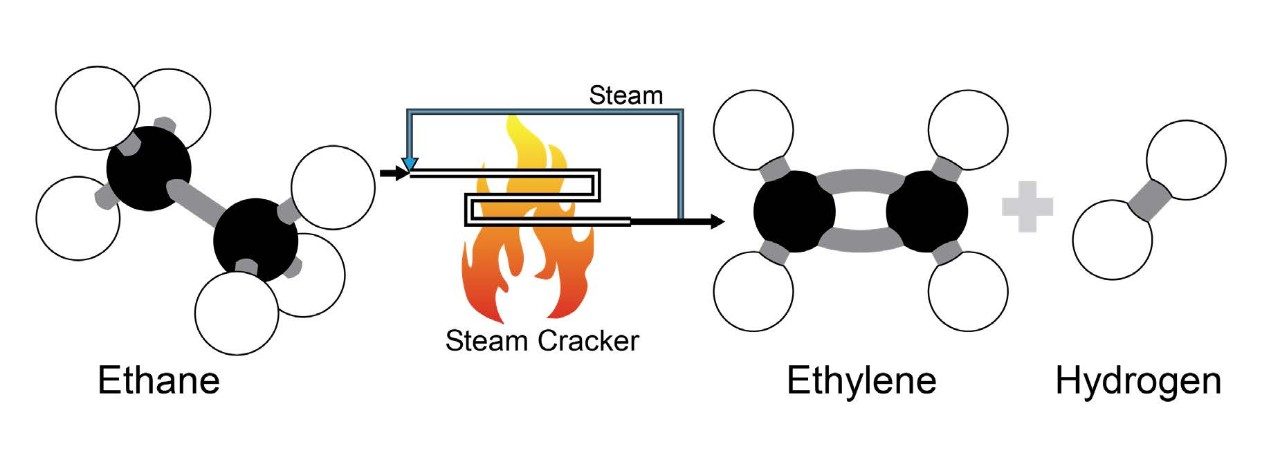
These deficiencies led Curme and his coworkers to consider the possibility of making ethylene from ethane, a component of abundantly available natural gas liquids. For this process, they developed a version of a technique known as thermal cracking, in which they heated ethane to a high temperature to convert it into ethylene and hydrogen. Curme and Pierre E. Haynes patented their method (#1,460,545).
Ethane cracking nearly took a dark turn in World War I, which the U.S. entered in April 1917. During his time at the Mellon Institute, Curme was approached by Raymond F. Bacon, director of the institute and technical head of the U.S. Army’s Chemical Warfare Service. Bacon was interested in developing a new source for synthesizing ethylene to manufacture dichloroethyl sulfide, or mustard gas, to support the U.S. war effort. Germany — which had a much more advanced chemical industry than the U.S. and could make mustard gas through a different route — was using the agent against British and other Allied forces to cause horrific blisters.
Bacon was aware of Curme’s work on the production of ethylene gas using the electric arc process, but Curme suggested using ethane instead because it was abundant and required much less energy to crack to produce ethylene.
Bacon agreed to Curme’s suggestion, and the Mellon Institute Prest-O-Lite fellowship began investigating processes to manufacture dichloroethyl sulfide derived from cracking ethane to produce ethylene. Using the resources of the Linde Company, a small-scale unit was set up in the Linde Laboratory in Buffalo, New York, to test the novel process. With its success, a small-scale commercial ethylene plant was set for construction in the summer of 1918 in Buffalo. Fortunately, the war ended in November of that year, effectively eliminating market demand for dichloroethyl sulfide.

High-resolution infographic
In the meantime, to convince Union Carbide management to develop a petroleum-based business, Curme submitted a prescient report, titled “The Possibilities of a Chemical Industry Based on the Simple Hydrocarbon Gases.” In his introduction, Curme stated: “In particular, ethylene, when complemented by acetylene and the by-products obtained in the production of these two substances from their various sources, provides the starting material for an organic chemical industry of almost unlimited proportions which might be extended as desired in any or all directions to cover a large part of the field of the existing chemical industry.”
As a result of his efforts, on Oct. 11, 1920, the Carbide and Carbon Chemicals Corporation was formed, and Curme was named manager and chief chemist. The goal of this new company was to commercialize production of several aliphatic compounds, or open-chain hydrocarbons, by developing a cost-effective process for manufacturing ethylene.
Just a few months before forming the subsidiary to manage the project, Union Carbide began looking for a production site in the Kanawha Valley of West Virginia, an appealing location because of the region’s abundance of hydrocarbon-rich gas as well as sufficient river and railroad transportation. The small gasoline extraction plant in Clendenin was purchased from the Clendenin Gasoline Company to serve as the facility for future research. In regard to the primitive condition of the facility, Curme stated, “It was a revelation that chemical processes functioned in a sheet-iron shack as well as under the best laboratory conditions.”
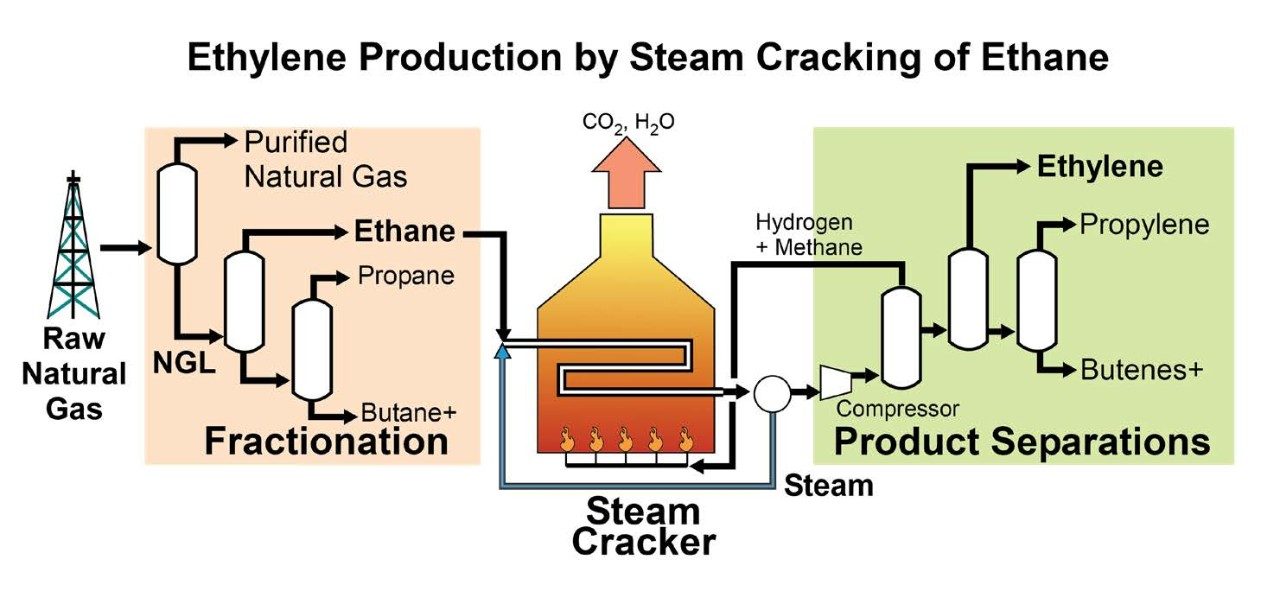
First, the Carbide engineers had to create an efficient method to evaporate the desired light hydrocarbons from natural gasoline so they could be captured rather than discarded. (Natural gasoline is similar to the gasoline commonly used in cars, but it is condensed from natural gas instead of being derived from oil.) The team designed a stabilizing column to separate and recover these compounds — primarily ethane and propane — from natural gasoline by distillation.
Next, a new system was developed to crack the recovered gases; it utilized a furnace and a separation “train” capable of separating and recovering the cracked gases. This process used the cracking technique patented by Curme and Haynes but was slightly modified to operate at higher temperatures. Rapidly heating ethane to temperatures exceeding 930 degrees Fahrenheit at a low pressure, only slightly above atmospheric conditions, produced high yields of ethylene. The hydrogen and methane by-products were burned to fuel the process. The ethylene was distilled to separate it from unreacted ethane, which was recycled.
Dr. George Oliver Curme is recognized as ‘the father, grandfather, and great-grandfather of ethylene and her numerous progeny.’" — “George Oliver Curme, Jr.,” a memoir by Augustus B. Kinzel
High-resolution infographic
The hydrocarbon separation plant and ethane cracking plant were completed in the summer of 1921. With this development, the Carbide and Carbon Chemicals Corporation created the first petrochemical plant that could separate light hydrocarbons and convert them directly into ethylene and its derivatives.
The next goal was to find markets for these newly developed chemicals. Curme started publishing articles that described the uses of ethylene and provided the necessary information to start marketing the new products.
The ethylene derivatives produced in Clendenin included ethylene dichloride, ethylene oxide, ethylene glycol, ethylene diacetate, and Cellosolve. Ethylene glycol was used in the manufacture of glycoldinitrate, a cost-effective substitute for nitroglycerine used in dynamite. Cellosolve is Union Carbide’s trade name for ethylene glycol monoethyl ether. This solvent was found to be ideal for nitrocellulose lacquers, which were used for painting automobiles. Another successful product developed in Clendenin was Pyrofax gas, bottled propane that could be used in homes for heating and cooking. Pyrofax became very popular and provided a large portion of the company’s income during early operations.
Selling the other products produced in Clendenin was much more difficult than anticipated. Curme and James Rafferty, a general manager at Union Carbide, went on sales trips with product samples packed in their suitcases; they would hand out specimens for a free trial in order to build a market for these newly developed products. In 1923, the company hired Joseph G. Davidson as the first salesman, and soon he and Curme became known as Carbide’s “Gold Dust Twins” because of their success. By 1934, over 50 derivative chemicals were being produced from the olefins made by cracking, launching the modern petrochemical industry.
Curme submitted a memo to the managers of Union Carbide stating: ‘In particular, ethylene, when complemented by acetylene and the by-products obtained in the production of these two substances from their various sources, provides the starting material for an organic chemical industry of almost unlimited proportions which might be extended as desired in any or all directions to cover a large part of the field of the existing chemical industry.’” — “George Oliver Curme, Jr.,” a memoir by Augustus B. Kinzel
But that success was still in the future back in 1923, when it was becoming clear that the company could scale up production, and it started surveying sites for a new location.
On Nov. 30 of that year, Union Carbide purchased a chemical plant in South Charleston, West Virginia, from the Rollins Chemical Company to serve as the new location for the first U.S. plant specifically designed to produce ethylene. This facility represented the culmination of the work done at the Mellon Institute in Pittsburgh; the Linde laboratory in Buffalo, New York; and the petrochemical plant in Clendenin, and it became a massive success.
Thermal cracking for ethylene production scaled well, and the wide range of products produced from ethylene led to larger, highly integrated sites. At these locations, multiple ethylene-derivative plants would be built around a cracker complex, driving down costs and opening new markets. The industry, which now involves many different companies, continues to evolve: Efforts are underway to make thermal cracking more sustainable using renewable energy supplied to electric furnaces.
Ethylene production — which topped 190 million tons worldwide in 2019 — forms the basis of today’s multi-billion-dollar chemical industry, making major contributions in the packaging, automobile, oil, medical, rubber, plastic, and other industries around the world, and providing products used in nearly every part of modern life.
The Carbide and Carbon Chemicals Corporation facilities that included the ethane cracking plant were located along the Elk River in Clendenin, now a residential area. The location of this site has a historical marker, along Route 119, that was erected in 1974 by the West Virginia Department of Archives and History. Entitled “Petrochemical Plant,” it states: “From this nucleus grew the nation’s giant petrochemical industry, employer of thousands.”

Booklet PDF
Landmark dedication
The American Chemical Society (ACS) honored Union Carbide’s foundation of the petrochemical industry with a National Historic Chemical Landmark (NHCL) in a ceremony in Clendenin, West Virginia, on Sept. 10, 2021. The commemorative plaque reads:
In the early 20th century, George Curme Jr. (1888-1976) realized that ethylene could supplement and eventually replace acetylene as the main building block for the emerging U.S. chemical industry. In 1921, Union Carbide began operating a facility in Clendenin to implement this vision. Using new separation and thermal cracking techniques, a Union Carbide team led by Curme isolated ethane from natural gas and converted it into ethylene at the facility. Union Carbide also commercialized processes for transforming ethylene into many other useful chemicals, thereby laying the foundation of today’s multi-billion-dollar global petrochemical industry. Ethylene-based compounds are now used in numerous everyday products – including clothing, packaging, detergents, paints, durable goods and construction materials.
Acknowledgements
Written by David Stone.
The author wishes to thank contributors to and reviewers of this booklet, all of whom helped to improve its content, especially members of the ACS NHCL Subcommittee.
The Kanawha Valley Section of the ACS, West Virginia State University, Union Carbide, and the Curme family sponsored the nomination for this Landmark designation.
Research resources
Further reading
- “A History of Union Carbide Corporation: From the 1890s to the 1990s,” by Robert D. Stief, Carbide Retiree Corps, 1998
- “Petrochemicals: The Rise of an Industry,” by Peter H. Spitz, 1988
- “Patent # 1,460,545: Production of Ethylene,” by Pierre E. Haynes and George O. Curme Jr., U.S. Patent Office, July 3, 1923
- “George Oliver Curme, Jr.,” a Biographical Memoir, by Augustus B. Kinzel, National Academy of Sciences, 1980
- “Revisiting the first cracker, in West Virginia, as shale rekindles interest,” Chemical Week, 2014

