Russell Marker and the Mexican Steroid Hormone Industry
Dedicated October 1, 1999, at Pennsylvania State University’s Pond Laboratory in University Park, Pennsylvania, USA, and December 2, 1999, at Syntex Laboratory in Mexico City, Mexico.
En español: Russell Marker y la industria mexicana de los esteroides
Steroid chemists often refer to the 1930s as the Decade of the Sex Hormones, when the molecular structures of certain sex hormones were determined and first introduced to medical practice as drugs. Russell Marker achieved the first practical synthesis of the pregnancy hormone, progesterone, by what now is known as the "Marker Degradation." Produced from starting material in a species of Mexican yam, Marker’s progesterone eventually became the preferred precursor in the industrial preparation of the anti-inflammatory drug cortisone. Important research on sex hormones continued in Mexico, leading to the synthesis of the first useful oral contraceptive in 1951.
Contents
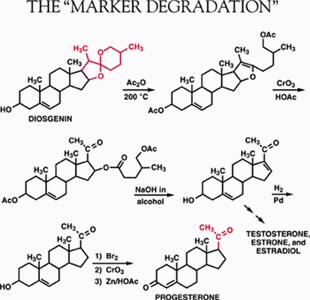
"Marker Degradation" and the Decade of the Sex Hormones
The Decade of the Sex Hormones — that's how steroid chemists often refer to the 1930s, when the molecular structures of the male hormone, testosterone, the female hormones, estrone and estradiol, and the pregnancy hormone, progesterone, were determined and first introduced to medical practice as drugs.
Much attention was focused on progesterone because of its value in treating various menstrual disorders and preventing certain types of miscarriages; however, its high cost severely restricted its use. The cost of progesterone and other important steroids fell drastically in the 1940s with the creation of the Mexican steroid industry. According to Carl Djerassi ("The Father of the Pill"), the "Mexican connection stemmed from the work of one gutsy individualist, Russell E. Marker," about whom more stories have been told, "many of them apocryphal… than about almost any other chemist."
In 1938, Marker, then a chemistry professor at The Pennsylvania State College (now, University), proposed a remarkable new molecular structure for sarsasapogenin, a plant steroid isolated from sarsaparilla. In Marker's structure, the side chain part of the molecule (the red part of the formula at right) is chemically reactive because two oxygen atoms are connected to the same carbon atom. Earlier workers had supposedly confirmed "beyond any doubt" a sarsasapogenin structure in which the side chain was chemically inert. Using this reactivity, Marker invented a chemical reaction sequence that removed most of the atoms in the side chain. What remained duplicated the side chain of progesterone. (Chemists call such processes "degradations.") Subsequent chemical modification of the steroid ring system then yielded progesterone itself.
Because of the high cost of sarsasapogenin and the major expense involved in these final manipulations, Marker began a search for a plant steroid of the sapogenin class with a ring structure more like progesterone. He found it in Beth root, a plant of the lily family, which already was being used in a patent medicine called "Lydia Pinkham's Compound." Japanese chemists previously had isolated this steroid from a yam of the Dioscorea family and named it diosgenin. As anticipated, when diosgenin was subjected to the "Marker Degradation," progesterone was obtained. However, neither Beth root nor the Japanese Dioscorea was an economical source of diosgenin.
In his search for a better diosgenin source, Marker recruited several botanists and launched extensive plant-collection trips mainly in the southwestern United States. More than 400 species were examined and a dozen new sapogenins were identified and characterized. Then, in November 1941, while in Texas, Marker was paging through a botany text when he saw the picture of a Dioscorea that grew in the Mexican state of Veracruz near Orizaba. The root of this plant, called cabeza de negro by the natives, was said to weigh up to 100 kilos.
What are Steroids?
Steroids are fat-soluble compounds with complex molecular structures that occur in almost all living organisms. They always have a ring system of carbon atoms with three 6-membered rings and one 5-membered ring connected to each other. The 5-membered ring can be attached to a side chain of carbon atoms that may be quite long (e.g., cholesterol and diosgenin), short (e.g., progesterone), or even nonexistent (e.g., testosterone, estrone, and estradiol).
Marker Discovers Mexican Dioscorea
When Marker went to Mexico City in January 1942, the U.S. Embassy advised him to leave immediately because of widespread anti-American sentiment fueled by World War II. Instead, Marker took a bus to Orizaba, changed to the local bus to Cordoba, and, on the way, recognized the stream described in the botany text. By the stream, he found a country store owned by Alberto Moreno, a native Mexican who did not speak English. Despite the language barrier, Moreno was enlisted to find some cabeza de negro. Although Marker had no plant-collecting permit, two large roots in bags soon were loaded on top of the bus to Orizaba. When Marker got there, the bags were gone, but he recovered the larger 50-pound root by bribing a local policeman.
Back at Penn State, Marker isolated diosgenin in satisfactory yield from part of the smuggled tuber. Because his research had been funded by Parke-Davis, Marker took the rest of his root to its laboratories in Detroit. There, he repeated his process in an attempt to persuade the company to commercialize it. However, Parke-Davis's president refused, because he believed chemical work could not be done in Mexico. Marker's efforts to interest other pharmaceutical houses also failed, and, by the fall of 1942, he was convinced the only path to success "was for me to do it myself."
Marker returned to Veracruz and arranged with Moreno to collect and dry about 10 tons of cabeza de negro. In Mexico City, he found a man with a small-scale extractor, who extracted the roots with alcohol and evaporated the extract to a syrup. Next, in return for a third of the product, Marker arranged with a New York friend, Norman Applezweig, to use his laboratory to convert the syrup to progesterone. Marker finished with three kilos valued at $80 per gram, then the largest lot of progesterone ever produced.
Founding Mexico’s Steroid Hormone Industry
Marker tried to interest Mexican entrepreneurs in exploiting his process. Eventually, while looking through the Mexico City telephone directory, he saw a listing for "Laboratorios Hormona, S.A.," a company set up in 1933 by Emeric Somlo and Federico Lehmann, primarily to produce gland extracts. When contacted, Lehmann recognized Marker's name, and because of his background in endocrinology, Lehmann also realized the significance of Marker's proposal. When Somlo arrived, the three agreed to form a company for the production of steroid hormones.
Marker ended his research program at Penn State during 1943 and resigned on December 1. He also told Parke-Davis he would only sign patent applications until that date. When the company delayed until April 1944, Marker refused to assign patent rights to anyone, including himself, thus granting free use of his invention to anyone interested. In early 1944, the new Mexican company was chartered and named Syntex, S.A. (from Synthesis and Mexico). According to Marker, Somlo was to receive 52% of the shares, Lehmann, 8%, and Marker, 40%, partly in return for his two kilos of progesterone. Working with four unskilled assistants in space provided by Hormona, Marker prepared his first kilo of progesterone by March. Within a year, Syntex was selling progesterone for $50 a gram.
In May 1945, a rancorous dispute between Marker and his partners over profits and their distribution caused Marker to sever all ties with Syntex and leave the company. Syntex was unable to make more progesterone because Marker not only had done the key operations himself but had coded the reagent bottles and left no directions.
By July, Marker was making progesterone in Texcoco, near Mexico City. His new company, Botanica-mex, was backed financially by Applezweig. Over the next months, several kilos of progesterone were synthesized, but production ceased in March 1946 because of the physical harassment of the workers by unidentified outsiders. Botanica-mex's assets were sold to Gedeon Richter Ltd. With Marker intermittently directing the work, this company started production in Mexico City under the name Hormonosynth. During this time, the cabeza de negro was replaced by another yam called barbasco, which contained five times as much diosgenin. After Marker retired, the company was reorganized as Diosynth.
After Marker left, Syntex began a frantic search for someone with the knowledge and expertise needed to get the company back into business. They found George Rosenkranz, who had studied at the Swiss ETH and was doing pharmaceutical research in Cuba. After an unusual job interview in which he actually performed a specific chemical reaction without directions, he was hired. A few months after Rosenkranz began work in October 1945, Syntex was again selling progesterone.
Rosenkranz extended the chemistry of diosgenin to the production of testosterone and other steroid hormones (also done by Marker). More importantly, Rosenkranz built a powerful research program at Syntex. In part, this was done by playing a major role in the creation of the "Instituto de Química," where a collaborative organic chemistry research degree was established. He also recruited other Ph.D. chemists for Syntex, including Carl Djerassi and Alejandro Zaffaroni.
Cortisone and the "Pill"
One research program led by Djerassi focused on the conversion of diosgenin to cortisone. Early in 1949, Philip Hench and Edward Kendall at the Mayo Clinic had reported spectacular results from the treatment of rheumatoid arthritis with cortisone. Newspapers featured stories of crippled arthritics dancing in the streets after therapy. The medical community soon recognized the value of cortisone (and cortisol) in the treatment of other inflammatory conditions, but it was too expensive for widespread use. At that time, cortisone could only be made by a laborious 36-step Merck & Co. process that started with desoxycholic acid isolated from ox bile. Syntex completed its synthesis of cortisone from diosgenin; however, this achievement immediately was overtaken by another scientific breakthrough.
In 1951, scientists at the Upjohn Co. introduced a microbiological process, which specifically oxidized progesterone to a product that was easily converted to cortisone. As Rosenkranz later recalled, "...in July 1951, I received a phone call from Upjohn asking me whether we would be able to accept an order for 10 tons of progesterone at 48 cents per gram. The going price at the time was in the $2 range and the quantity was unheard of." Soon, most cortisone was manufactured by Upjohn's process using Syntex progesterone, although the Merck route was improved enough to remain competitive.
Syntex also competed with other drug companies in the hunt for an effective oral contraceptive. Because progesterone prevents ovulation during pregnancy, research focused on the discovery of an orally active progesterone mimic. Using expertise obtained in the conversion of testosterone to estradiol and estrone, along with two important leads from the chemical literature, Djerassi's group designed and, in 1951, synthesized norethindrone, the active ingredient in the first practical oral contraceptive. For many reasons, the first "pill" to be marketed was G. D. Searle and Co.'s norethynodrel, but norethindrone accounted for more than half of the oral contraceptive market in the 1970s.
By the 1950s, Syntex and its competitors in Mexico* were producing more than half the sex hormones sold in the United States. Syntex's economic success was matched by its scientific reputation, which was boosted by a very liberal publication policy. Contributions made by Syntex accounted for more than 30% of all industrial citations in the Fiesers' definitive 1959 monograph, Steroids. In 1951, Fortune magazine headlined: "Syntex makes the biggest technological boom ever heard south of the border." Considering the impact the Mexican steroid industry would have on world health and culture, Fortune greatly underestimated the power of the explosion.
*Besides Diosynth and others, these competitors included Glidden and, later, Laboratories Julian, the independent institute of Percy L. Julian, Glidden's former research director. Julian's starting material was stigmasterol, a steroid isolated from soybean oil. Learn more about Julian's work by visiting the Percy L. Julian and the Synthesis of Physostigmine National Historic Chemical Landmark.
Biography of Russell Marker
Russell Earl Marker was born on his father's farm near Hagerstown, Maryland, on March 12, 1902. After receiving a B.S. degree in 1923 from the University of Maryland and an M.S. degree in physical chemistry in 1924, he began doctoral research with Morris Kharasch. Within a year, Marker had completed enough work for his thesis but was told he still needed to take some required physical chemistry courses, which he considered a waste of time. Kharasch predicted that he would end up as a "Urine Analyst" if he didn't complete these Ph.D. requirements, but Marker "accepted his challenge and left the university in June 1925." Later, Kharasch officially approved Marker's thesis on organomercurials and quaternary alkyl hydrocarbons. Although Marker never received a Ph.D. from Maryland, the university awarded him an Honorary Doctor of Science in 1987.
In 1926, Marker married Mildred Collins (1899-1985) and began work at the Ethyl Corp. There, he invented the gasoline "octane number" rating system and, in the process, discovered that increased branching in hydrocarbons reduced knocking. The oil refining companies immediately retooled their processes to maximize branch chain hydrocarbons to improve gasoline quality.
By 1928, Marker believed he had enough hydrocarbon experience and began research with P. A. Levene at the Rockefeller Institute. Over the next six years, Marker did enough research for 32 papers on optical rotation and molecular configurations. By 1934, Marker wanted to change his focus to steroid research. When Levene refused, Marker accepted a position funded by Parke-Davis at The Pennsylvania State College.
During 1936-1937, Parke-Davis sent Marker a steroid extract from the urine of pregnant cows and mares. From this, he isolated pregnanediol, which he converted by known chemistry to 35 grams of progesterone (then, the most ever produced in one lot). Over the next six years at Penn State, Marker and his small research group did most of his celebrated research. Parke-Davis provided annual funding that eventually reached $10,000. Ultimately, more than 160 papers in the steroid area were published. In 1944, Marker cofounded Syntex and, in 1945, Botanica-mex. In 1949, his report that botogenin should be the best cortisone precursor received wide publicity.
Soon after that, Marker retired from chemistry and essentially disappeared. Although sporadic sightings occurred, he often was reported to be dead or "in an insane asylum." After retirement, Marker actually spent most of his time in Mexico City and at home in State College, Pennsylvania. He became interested in three great 18th-century silversmiths and began commissioning Mexican reproductions of their works. To guarantee that each piece was correct, he spent much time in museums and other collections.
Marker reentered public life in 1969 to accept an award from the Mexican Chemical Society at the VI International Symposium on the Chemistry of Natural Products in Mexico City. Other awards followed, including one at the 1975 Chemical Congress of North America. Marker also was rediscovered by the popular press and featured in articles, documentaries, and even a 90-minute German TV biographical "docudrama." In later life, his philanthropies included an endowed professorship at Penn State and several endowed lecture series at Penn State and the University of Maryland. Marker died March 23, 1995.
Further Reading
- Information about Russell Marker (Pennsylvania State University)
- Sociedad Química de México (Chemical Society of Mexico)
- Biography of Russell E. Marker (Chemical Heritage Foundation)
- En español: Russell Marker y la industria mexicana de los esteroides (ACS)
Landmark Designation and Acknowledgments
Landmark Designation
The American Chemical Society and Sociedad Química de México dedicated the "Marker Degradation" and the creation of the Mexican steroid hormone industry as an International Historic Chemical Landmark in 1999.
The text of the plaque dedicated on October 1, 1999, at the Pond Laboratory at Pennsylvania State University in University Park, Pennsylvania, USA, reads:
In Pond Laboratory, Russell E. Marker achieved the first practical synthesis of the pregnancy hormone, progesterone, by what now is known as the "Marker Degradation." After discovering an economical source of his starting material in a species of Mexican yam, Marker commercialized his process in 1944 at Syntex, S.A., which he founded in Mexico City with Emeric Somlo and Federico A. Lehmann. This low-cost progesterone eventually became the preferred precursor in the industrial preparation of the anti-inflammatory drug cortisone. In 1951, Syntex researchers synthesized the first useful oral contraceptive from Marker's starting material. Syntex and its competitors in Mexico thus became a powerful international force in the development of steroidal pharmaceuticals.
The English version of the text of the plaque dedicated on December 2, 1999, at the site of the Syntex laboratory in Mexico City, Mexico, reads:
In 1944, at this first site of Syntex, S.A., in Mexico City, Pennsylvania State College chemist Russell E. Marker accomplished the first industrial conversion of diosgenin (from a Mexican yam) to the pregnancy hormone, progesterone, using chemical reactions now known as the "Marker Degradation." Marker, along with Emeric Somlo and Federico A. Lehmann of Laboratorios Hormona, founded the new company. The low-cost progesterone eventually became the preferred precursor in the industrial preparation of the anti-inflammatory drug cortisone. In 1951, Syntex researchers synthesized the first useful oral contraceptive from Marker's starting material. Syntex and its competitors in Mexico thus became a powerful international force in the development of steroidal pharmaceuticals.
Acknowledgments
Adapted for the internet from “The ‘Marker Degradation’ and Creation of the Mexican Steroid Hormone Industry 1938-1945,” produced by the National Historic Chemical Landmarks program of the American Chemical Society in 1999.
Back to National Historic Chemical Landmarks Main Page
Learn more: About the Landmarks Program
Take action: Nominate a Landmark and Contact the NHCL Coordinator
Teach: Landmarks Lesson Plans







