What molecule am I?
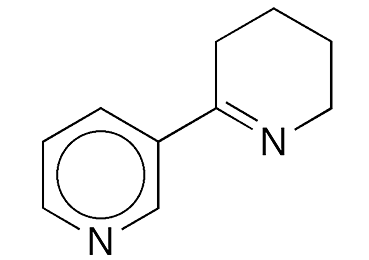
Anabaseine is a bipyridine derivative and piperidine analogue of anabasine1, the Molecule of the Week for December 29, 2008. Both molecules are chemically related to nicotine, the August 20, 2018, MOTW.
Anabaseine was first synthesized in 1936 by E. Späth* and L. Mamoli at the University of Vienna via a Claisen condensation. In 1971, William R. Kem*, Bernard C. Abbott*, and Robert M. Coates at the University of Illinois at Champaign–Urbana reported the isolation of anabaseine from a nemertean (ribbon worm), which uses the molecule to paralyze prey and repel predators. In 1981, J. W. Wheeler, R. M. Duffield, and co-workers at Howard University (Washington, DC) isolated it from the poison glands of Aphaenogaster ants, which use anabaseine as a pheromone and a chemical defense.
Because of its chemically reactive imine group, anabaseine is a useful scaffold for synthesizing nicotinic receptor drug candidates. These include GTS-212 (synthesized by Kem), which has been clinically tested to improve cognition in schizophrenics and pre–Alzheimer’s disease patients.
In 2009, Palmer Taylor, Yves Bourne, and coauthors at the University of California, San Diego, the University of Aix-Marseille (France), and the University of Florida (Gainesville) reported on the interactions of GTS-21 and several other substituted anabaseines with molluscan acetylcholine-binding proteins. The following year, a quantitative structure–activity relationship analysis of benzylidene–anabaseines by Kem, Alan R. Katritzky, and co-workers at the University of Florida provided additional insights concerning ligand–nicotinic receptor interactions.
1. CAS Reg. No. 494-52-0.
2. CAS Reg. No. 156223-05-1.
Anabaseine hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
In 2009, Ileana I. Rodríguez*, Abimael D. Rodríguez, and Hong Zhao at the University of Porto Rico (San Juan) described the discovery of (+)-aberrarone1, a product of the gorgonian-type (soft) West Indian coral Pseudopterogorgia elisabethae. The molecule has a 5-5-5-6 ring system with four ketone groups, three quaternary carbons, and seven stereogenic centers, six of which are contiguous.
Then, this past August, Willi M. Amberg and Erick M. Carreira* at ETH Zurich described a 15-step total synthesis of this unusual molecule. In one step, the researchers used a tin alkoxide cocatalyst to improve the performance of a gold-catalyzed reaction.
Chloramine2 (NH2Cl), also called monochloramine, is an unstable, corrosive liquid often used in small amounts as a disinfectant for drinking water and swimming pools. Chloramine has been known since the early 20th century; German chemist Friedrich Raschig described its chemistry in 1908.
This August, Baoyou Shi and coauthors at the Chinese Academy of Sciences (Beijing) and Zhuhai Water Environment Holdings Group (Guangdong, China) reported another use for chloramine. In their efforts to combat the microbial oxidation of dissolved manganese(II) to MnOx deposits in drinking water lines, they examined the use of chlorine treatments. They found that simple chlorination did not prevent the problem, but the use of chloramine prevented almost all MnOx accumulation.
1. CAS Reg. No. 1187225-09-7.
2. CAS Reg. No. 10599-90-3.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Anabaseine fast facts
| CAS Reg. No. | 3471-05-4 |
| SciFinder nomenclature | 2,3′-Bipyridine, 3,4,5,6-tetrahydro- |
| Empirical formula | C10H12N2 |
| Molar mass | 160.22 g/mol |
| Appearance | Colorless oil |
| Boiling point | 110–120 °C |
| Water solubility | Slighta |
a. Very soluble as its dipicrate, dihydrochloride, or diperchlorate salt.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

