What molecule am I?
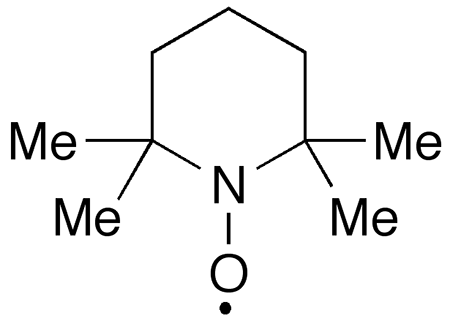
TEMPO, like DABCO, the Molecule of the Week for August 29, 2022, is one of those molecules best known by its acronym. TEMPO’s full name is 2,2,6,6-tetramethyl-1-piperidinyloxy.
TEMPO is also unusual in that it is a stable free radical. Because of its unpaired electron, its color is a bright red-orange, in contrast to similar molecular structures, which do not absorb light in the visible region.
TEMPO was first synthesized in 1959 by USSR chemists O. L. Lebedev and S. N. Kazarnovskii. They oxidized 2,2,6,6-tetramethylpiperidine1 with hydrogen peroxide in the presence of pertungstate ion (WO42–). The authors called the resulting product an “intermediate”; it is not clear whether they realized that it was a free radical. Three years later, another Soviet researcher, A. V. Il’yasov, published data on the effect of solvents on the electron paramagnetic resonance2 (EPR) spectra of some stable free radicals, including TEMPO, so its status had been established by then.
TEMPO has multiple uses in organic chemistry: as a catalyst in oxidation reactions and radical-mediated polymerizations and as an EPR marker or probe in chemical and biochemical systems. In a recent example, Abudureheman Wusiman and co-workers at Xingjiang Normal University (Urumqi, China) used it to promote the mono- and bisimidation of tertiary anilines. The purpose of the reaction was to create a straightforward route to symmetric and unsymmetric N-Mannich bases3 under mild conditions.
1. CAS Reg. No. 768-66-1.
2. Also known as electron spin resonance (ESR).
3. A Mannich base is a β-amino ketone; an N-Mannich base has a nitrogen atom in place of the intermediate carbon atom, e.g., R2NCH2NHC(O)R.
TEMPO hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Skin corrosion/irritation, category 1B | H314—Causes severe skin burns and eye damage | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Short-term (acute) aquatic hazard, category 3 | H402—Harmful to aquatic life | |
| Long-term (chronic) aquatic hazard, category 3 | H412—Harmful to aquatic life with long-lasting effects | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
6,6′-Biphenanthridine1 (biphe) is a benzannulated derivative of 2,2′-bipyridine that was first described in 1961 by John J. Eisch* at the University of Michigan (Ann Arbor) and Robert M. Thompson at Saint Louis University. In the solid state, the two phenanthridine moieties are not coplanar because of steric hindrance. In solution, biphe displays blue fluorescence; its monoprotonated form fluoresces yellow.
This past August, J. A. Gareth Williams, David E. Herbert, and coauthors at the University of Manitoba (Winnipeg) and Duke University (Durham, NC) reported the synthesis, characterization, and coordination chemistry of biphe. In particular, biphe coordination complexes with ruthenium(II) are potential ingredients for deep-red phosphors.
Echinosulfonic acid D2 is a cytotoxic bis(bromoindole) alkaloid that occurs in a New-Caledonian sea sponge in the Psammoclemma genus. It was isolated in 2005 by Thierry Sévenet and collaborators at the Institute of Chemistry of Natural Substances, CNRS (Gif-sur-Yvette) and the Center on Natural Substances Research (Ramonville, both in France).
In 2020, Gavin R. Flematti and colleagues at the University of Western Australia (Crawley) and the Western Australian Museum (Welshpool) used single-crystal X-ray diffraction and density functional theory analysis to reassign the structures of echinosulfonic acids A–D3. This August, Takumi Abe, Daisuke Sawada, and co-workers at Okayama University (Japan) reported the first total synthesis of the reassigned structure of echinosulfonic acid D.
1. CAS Reg. No. 75164-08-8.
2. CAS Reg. No. 851983-54-5.
3. Echinosulfonic acids A–C occur in an Australian marine sponge of the Echinodictyum genus.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
TEMPO fast facts
| CAS Reg. No. | 2564-83-2 |
| SciFinder nomenclature | 1-Piperidinyloxy, 2,2,6,6-tetramethyl- |
| Empirical formula | C9H18NO |
| Molar mass | 156.25 g/mol |
| Appearance | Dark red to red-orange crystals or powder |
| Melting point | 36–38 °C |
| Water solubility | 12 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

