What molecule am I?
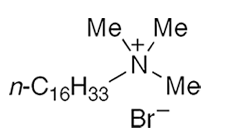
Cetyltrimethylammonium bromide (CTAB), sometimes called cetrimonium bromide, is a quaternary ammonium salt with surface-active and antiseptic properties. It was mentioned in the chemical literature as early as 1936, when G. S. Hartley*, B. Collie, and C. S. Samis at University College London studied transport numbers of paraffin-chain salts in aqueous solutions.
In 1946, R. S. Shelton and co-workers at the William S. Merrell Company1 (Cincinnati) reported a synthesis of CTAB from cetyl (hexadecyl) bromide and trimethylamine. They prepared CTAB and several other compounds during a study of the efficacy of quaternary ammonium salts as what they termed “germicides”.
Today, in addition to its use as a topical antiseptic, CTAB has applications in medicine as an apoptosis-promoting anticancer agent, and in protein electrophoresis, DNA extraction buffer systems, and nanoparticle synthesis. In a report this month, Jonathan Boltersdorf, Taylor J. Woehl, and coauthors at the University of Maryland (College Park), the Army Research Laboratories (Adelphi, MD), General Technical Services (Wall Township, NJ), and the Naval Surface Warfare Center (Crane, IN) used CTAB as a surfactant in their examination of silver photodeposition onto gold nanorods.
1. After a long series of mergers and acquisitions, William S. Merrell is now part of Sanofi.
CTAB hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Specific target organ toxicity, repeated exposure, oral, gastrointestinal tract, category 2 | H373—May cause damage to organs (gastrointestinal tract) through prolonged or repeated exposure if swallowed | |
| Short-term (acute) aquatic hazard, category 1 | H400—Very toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 1 | H410—Very toxic to aquatic life with long-lasting effects | |
*Compilation of two safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule of the Future
Analgesics, or pain killers, function as agonists for adenosine A1 receptors (A1Rs), which are ubiquitous in the body. But conventional analgesics, including opioids, have undesirable side effects, such as sedation, bradycardia (slow heart rate), hypotension, and respiratory depression.
Last July, Mark J. Wall, Graham Ladds, Bruno G. Frengueli, and collaborators at the Universities of Warwick and Cambridge (both in the UK) and other institutions reported that, in animal testing, the molecule benzyloxycyclopentyladenosine1 (BnOCPA) is a selective agonist for A1Rs that elicits analgesia without cardiorespiratory depression. Unlike opioids, BnOCPA is nonaddictive and has few side effects.

The synthesis of BnOCPA was first described in a 2005 US patent application by inventors Elfatih Elzein and Jeff Zablocki, assigned to CV Therapeutics (Palo Alto, CA).
1. CAS Reg. No. 872693-38-4.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
CTAB fast facts
| CAS Reg. No. | 57-09-0 |
| SciFinder nomenclature | 1-Hexadecanaminium, N,N,N-trimethyl-, bromide |
| Empirical formula | C19H42BrN |
| Molar mass | 364.45 g/mol |
| Appearance | White crystals or powder |
| Melting point | 248–251 °C |
| Water solubility | 36 g/L |
MOTW update
Xylan was the Molecule of the Week for August 8, 2022. It is a hemicellulose, one of the three types of fibers found in plants. This month, Bjørge Westereng, Kirsi S. Mikkonen, Tiina Nypelö, and colleagues at three Scandinavian universities reviewed the possibilities of using wood hemicelluloses, including xylan, as food ingredients. Among other uses, they report that wood hemicelluloses are structurally similar to those obtained from food crops and explore their potential as dietary fibers. Hemicelluloses from agricultural waste also can be used to manufacture valuable products such as chemicals and packaging materials.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

