What molecule am I?

Writing in the Encyclopedia of Biological Chemistry II, biochemist Judith Ann Foster of Boston University describes elastin as
Elastin takes many forms and compositions depending on its location in the organism. In a 1938 report on the composition of elastin, William A. Stein and Edgar G. Miller, Jr., at Columbia University (New York City) identified and quantified 10 amino acids, the most abundant of which were glycine and leucine.
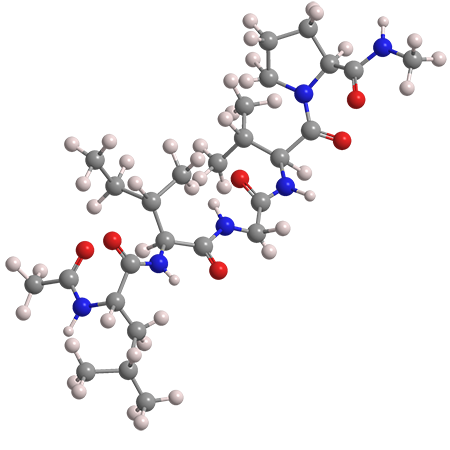
The images show one of the common structures within elastin: the peptide chain N-acetyl-L-leucyl-L-isoleucyl-glycyl-L-valyl-L-proline methylamide. Although components of elastin, called tropoelastins, are water-soluble, the large elastin protein is not.
In some respects, elastin is similar to titin, the Molecule of the Week for November 7, 2022. In humans, titin is encoded by the TTN gene in chromosome 2; elastin is coded by ELN in chromosome 7.
For additional information on elastin, see ScienceDirect’s abstract page.
Elastin hazard information
| Hazard class* | GHS code and hazard statement |
|---|---|
| Not a hazardous substance or mixture |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
MOTW updates
Hydrogen peroxide (H2O2) was the Molecule of the Week for January 26, 2007. It was updated in August 2022 with two articles describing the use of the oxygen reduction reaction to prepare H2O2. This past week, another H2O2 synthesis appeared: Siji Ogo and co-workers at Kyushu University (Fukuoka, Japan) described a one-pot, homogeneously catalyzed reaction between oxygen and hydrogen in aqueous solvent to make H2O2 under ambient conditions. The process uses a rhodium-based catalyst system and avoids explosive H2–O2 mixtures.
Carbon nitride (C3N4) was the Molecule of the Week for January 16, 2023. Among its several allotropes, β-C3N4 has properties similar to those of diamond, whereas the properties of g-C3N4 are more like graphite. g-C3N4 has valuable semiconductor and photocatalytic properties; but earlier this month, Hanning Chen, Danmeng Shuai, and colleagues at the University of Texas at Austin, the George Washington University (Washington, DC), and four other institutions reported that g-C3N4 nanosheets are readily oxidized by reactive chlorine species (RCS). The authors found that the free radicals ClO∙ and Cl2∙– decompose the sheets more rapidly than other RCS.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Elastin fast facts
| CAS Reg. No. | 9007-58-3 |
| SciFinder nomenclature | Elastins |
| Empirical formula | C27H48N6O6 (component) |
| Molar mass | 552.71 g/mol (component) |
| Appearance | Pale yellow fibers |
| Melting point | N/A |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

