What molecule am I?
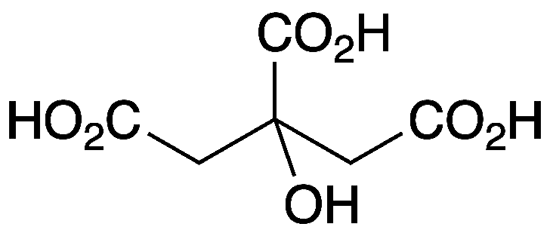
Citric acid is an important natural compound that has been known since the late 18th century. The pioneering Swedish–German chemist Carl Wilhelm Scheele isolated it from lemon juice in 1784. It has since been found in other citrus fruits, pineapples, and even animal tissues.
Citric acid is a major industrial chemical, produced at >2 million t/year worldwide. Its main source is not from fruit, but from the fermentation of crude sugars (e.g., molasses and corn starch) by the mold Aspergillus niger. It has a myriad of uses, mostly in foods and pharmaceuticals; these uses include acidifying agent/pH adjustment, antioxidant, flavoring agent, and as metal salts in dietary supplements. In industry and domestic applications, citric acid is a chelating and buffering agent in many cleaning products and a starting material for synthesizing citrate esters, itaconic acid, acetonedicarboxylic acid, and other compounds.
Biochemists are familiar with the citric acid cycle, which is a major life process in all respiring organisms. Also called the Krebs cycle or the tricarboxylic acid cycle, the process begins with sugar-derived pyruvate, which enzymatically generates acetyl-coenzyme A (CoA) to start the cycle. Acetate released from acetyl-CoA reacts with oxaloacetic acid produced at the end of the previous cycle to form citric acid; this is followed by several steps, during which an oxidation reaction releases energy to the body in the form of adenosine triphosphate.
Many scientists contributed to the discovery and establishment of the citric acid cycle. The two key researchers were Albert Szent-Györgyi at the University of Szeged (Hungary) and Hans Adolf Krebs at the University of Sheffield (UK); they were awarded the Nobel Prize in Physiology or Medicine in 1937 and 1953, respectively.
Citric acid hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Compilation of multiple safety data sheets. The most severe reported hazards are shown.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule that you would like us to consider, please send us a message, preferably including links to references about your suggested molecule. And thank you for your interest in Molecule of the Week! —Ed.
Citric acid fast facts
| CAS Reg. No. | 77-92-9 |
| SciFinder nomenclature | 1,2,3-Propanetricarboxylic acid, 2-hydroxy- |
| Empirical formula | C6H8O7 |
| Molar mass | 192.12 g/mol |
| Appearance | Colorless or white crystals or powder |
| Melting point | 153 °C |
| Water solubility | 1.33 kg/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.


