What molecule am I?
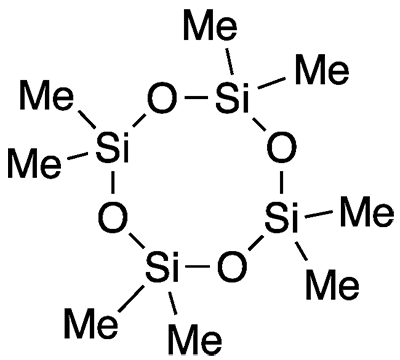
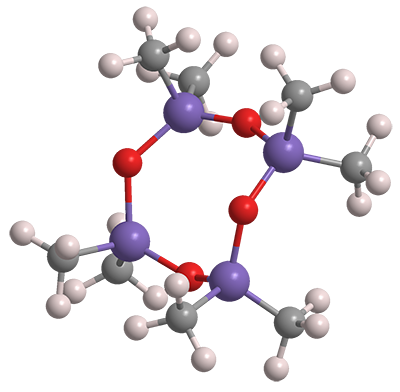
Octamethylcyclotetrasiloxane is a member of the cyclic siloxane, or cyclomethicone, family. It is often called simply D4 because of its four dimethylsiloxane units. It exists as an oily liquid or low-melting solid.
In 1946, Winton Patnode and Donald F. Wilcock at General Electric (Schenectady, NY) prepared D4 as part of an extensive study of methylpolysiloxanes made by hydrolyzing methylchlorosilanes. In one the many reactions they reported, they mixed dimethyldichlorosilane1 (Me2SiCl2) with large volumes of water to produce a colorless oil with a composition that had an empirical formula close to that of Me2SiO.
Analysis of the oil showed that it was primarily a mixture of cyclic oligomers of the formula (Me2SiO)x, with isolated compounds ranging from x = 3 to x = 10. The relative amounts of each of the cyclosiloxanes varied with hydrolysis conditions such as the Me2SiCl2/H2O ratio and the presence of cosolvents, acids, or bases. In general, D4 (x = 4) was the most abundant product.
Today, D4 is commercially produced by the same general method. The product mixture is separated into its components via distillation. D4 and the other oligomers are used to prepare poly(dimethylsiloxane)2 [(Me2SiO)n], familiarly known as silicone oil. D4 itself is used as an additive to plastic and rubber products, paints, adhesives, cosmetics, food packaging, and many other product areas. Its worldwide production is in the hundreds of thousands of tonnes.
Because of its widespread production and use, D4 has come under scrutiny for its adverse environmental and health effects. Last September, the US Environmental Protection Agency announced an evaluation of the environmental risks of its industrial uses. It will be up to the Food and Drug Administration to determine whether its food, health care, and personal care uses are evaluated.
1. CAS Reg. No. 75-78-5.
2. CAS Reg. No. 9016-00-6.
Octamethylcyclotetrasiloxane hazard information
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 1 | H224—Extremely flammable liquid and vapor | |
| Acute toxicity, oral, category 4 | HH302—Harmful if swallowed | |
Acute toxicity, dermal, category 3 | H311—Toxic in contact with skin | |
| Reproductive toxicity, category 2 | H361—Suspected of damaging fertility or the unborn child | |
| Long-term (chronic) aquatic hazard, category 3 | H413—May cause long-lasting harmful effects to aquatic life | |
*Compilation of multiple safety data sheets. The most severe reported hazards are shown.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Octamethylcyclotetrasiloxane
fast facts
| CAS Reg. No. | 556-67-2 |
| SciFinder nomenclature | Cyclotetrasiloxane, 2,2,4,4,6,6,8,8-octamethyl- |
| Empirical formula | C8H24O4Si4 |
| Molar mass | 296.62 g/mol |
| Appearance | Colorless oily liquid or white solid |
| Melting point | 17–18 °C |
| Boiling point | 175–176 °C |
| Water solubility | 56 µg/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

