What molecule am I?
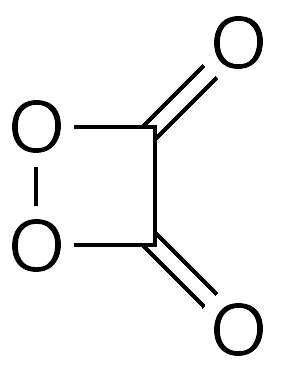
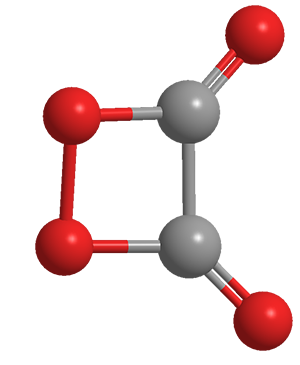
1,2-Dioxetanedione is a highly strained four-membered ring molecule that can be thought of as a peroxy anhydride of oxalic acid or as a dimer of carbon dioxide. Because of its high strain, it is quite unstable, decomposing at temperatures above –93 °C.1
Other than its unique structure, 1,2-dioxetanedione has another claim to fame: its participation in a reaction sequence that leads to chemiluminescence, a process that produces light via a chemical reaction. Chemiluminescence that results from the reaction between oxalyl chloride or an oxalate ester and hydrogen peroxide in the presence of an activator dye has been studied for almost 60 years, but only recently was it demonstrated that 1,2-dioxetanedione is the key high-energy intermediate (HEI).
In 2021, Wilhelm J. Baader at the University of São Paulo (Brazil) and colleagues there and at the Federal University of ABC (Santo André, Brazil) conclusively showed that the HEI is indeed 1,2-dioxetanedione. Using various arylperoxyoxalate esters to generate the HEI and precise kinetic and thermodynamic measurements, the researchers concluded that 1,2-dioxetanedione’s planar structure and electron-acceptor properties made it the only participant in the reaction sequence capable of being the HEI. Their experiments eliminated other known intermediates, such as peroxalic acids and 1,2-dioxetanones, from having enough energy to drive the luminescence step.
What are the practical applications of 1,2-dioxetanedione and chemiluminescence? Certainly, the best known are the novelty products known as glowsticks. Back in the 1960s, a research manager at the now-defunct American Cyanamid (Wayne, NJ) noticed an article by Edwin A. Chandross at Bell Laboratories (Murray Hill, NJ; now Nokia Bell Labs) that first described the phenomenon. Cyanamid chemists refined Chandross’s reaction sequence into commercial glowsticks, which were the basis for a spinoff company, Cyalume Technologies (West Springfield, MA).
At about the same time, the US Department of Defense and the military developed glowsticks for such uses as training devices and life-saving flotation vests. Innovations at Cyalume since then have brought forth glowsticks with chemiluminescence lifetimes from 30 sec to 24 h and with operating temperatures ranging from –50 to +50 °C.
1. Another possible CO2 dimer, 1,3-dioxetanedione (CAS Reg. No. 175600-75-6), is thought to be even less stable than the 1,2-isomer.
Molecule of the future
Spiroaxillarone A1 is a naturally occurring spirobisnaphthalene that Florian T. Schevenels and co-workers at Khon Kaen University (Thailand) isolated in 2019 from the flowering plant Cyanotis axillaris, which grows in India, China, Southeast Asia, and Australia. The authors noted that
- the molecule’s spirobisnaphthalene structure is the first found in nature;
- the molecule is both symmetrical and chiral; and
- it shows significant activity against Plasmodium falciparum, the protozoan parasite that causes malaria in humans.
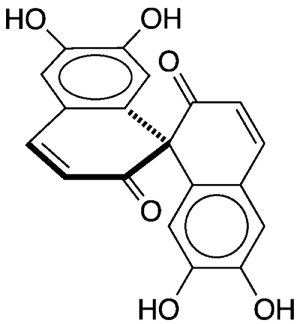
Within 2 years after the Schevenels team’s discovery, two research groups reported the total synthesis of racemic spiroaxillarone A. The first, Zhixiang Xie and colleagues at Lanzhou University (China), prepared the compound in five steps and 10% overall yield from tetrahydrocurcumin2, a metabolite of naturally occurring curcumin. They formed the spiro ring structure via an oxidative free-radical cycloaddition.
In the second synthesis, Jun Deng at Nankai University (Tianjin, China) and coauthors at Yunnan University (Kunming, China) and Kunming Institute of Botany used what they described as a de novo, bioinspired route to (±)-spiroaxillarone A. Their sequence consisted of six steps and 22% overall yield starting from commercially available 3,4-dihydroxybenzaldehyde3; the key step was a sulfa-Michael addition reaction.
1. CAS Reg. No. 2411027-11-5.
2. CAS Reg. No. 36062-04-1.
3. CAS Reg. No. 139-85-5.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
1,2-Dioxetanedione
fast facts
| CAS Reg. No. | 26974-08-3 |
| SciFinder nomenclature | 1,2-Dioxetane-3,4-dione |
| Empirical formula | C2O4 |
| Molar mass | 88.02 g/mol |
| Appearance | N/A |
| Melting/boiling point | –93 °C (dec.) |
| Water solubility | N/A |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

