What molecule am I?
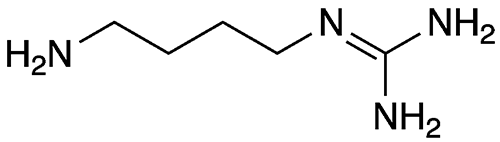
Agmatine, aka 1-(4-aminobutyl)guanidine, is a natural substance that is formed by the decarboxylation of the amino acid l-arginine, the Molecule of the Week for September 25, 2017. Agmatine belongs to a group of molecules called biogenic amines (BAs), which includes former MOTWs histamine, tyramine, putrescine, cadaverine, spermidine, and spermine. Agmatine and other BAs are particularly abundant in high-protein foods such as meats and dairy products.
In 1910, biochemist Albrecht Kossel at the Heidelberg Academy of Sciences (Germany) discovered agmatine in herring roe and reported a synthesis of it. Kossel was awarded the Nobel Prize in Physiology or Medicine the same year for his discoveries in cell biology. Nine years later, in an extensive study, Frederick W. Heyl at Upjohn (Kalamazoo, MI) reported the presence of agmatine in pollen protein extracts from ragweed (Ambrosia artemisiifolia).
Agmatine has a variety of biochemical properties. It can modify neurotransmitter systems, ion channels, nitric oxide (NO) synthesis, and polyamine metabolism. In a 1994 article, G. Li and co-workers at Cornell University Medical College (New York City) reported that agmatine is formed in the bovine brain and displaces clonidine by binding to α-2-adrenergic and imidazoline receptors. They also state that agmatine may act as a neurotransmitter.
Agmatine is is described as an important biomolecule in the 2021 book Starving Cancer Cells: Evidence-Based Strategies to Slow Cancer Progression by Robert Fried, Richard M. Carlton, and Dennis A. Fried. They cite a study in which agmatine abolished ornithine decarboxylase protein expression and polyamines biosynthesis to induce caspase-dependent apoptosis, and another that showed that it inhibited NO production mainly by depressing inducible NO synthase activity.
Agmatine hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Agmatine fast facts
| CAS Reg. No. | 306-60-5 |
| SciFinder nomenclature | Guanidine, N-(4-aminobutyl)- |
| Empirical formula | C5H14N4 |
| Molar mass | 130.19 g/mol |
| Appearance | White crystals or powder |
| Melting point | 102 °C |
| Water solubility | ≈13 g/L |
MOTW update
Imidacloprid1 was the Molecule of the Week for August 25, 2014. It is a neonicotinoid pesticide that has come under regulatory pressure because of its neurotoxic risk to honeybees.
In 2020, the US Environmental Protection Agency unexpectedly announced that it was allowing the continued use of neonicotinoids, including imidacloprid. But the pressure to restrict them continues. Last month, the California Department of Pesticide Regulations (DPR) announced it will ban the use of neonicotinoids on flowering crops when they are in bloom. DPR estimates that this will reduce neonicotinoid use by ≈45% in California.
1. CAS Reg. No. 138261-41-3.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

