What molecule am I?
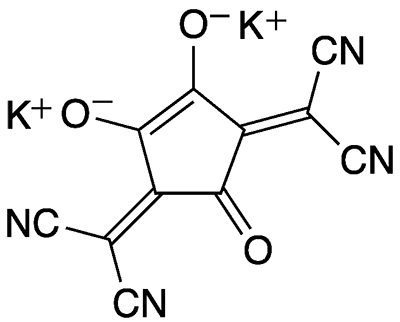
Croconate violet1 is a member of the croconate family of salts of cyclic unsaturated oxocarbons. Croconate itself is a five-membered ring with five oxygen atoms and a 2– charge. Croconate violet has dicyanomethylene groups in place of two of the oxygen atoms; but it retains the trivial name “croconate”.
Despite the structures shown, croconate violet’s π-electrons, like those in all croconates, are completely delocalized and are responsible for the molecules’ distinctive properties. Croconate violet was named by Alexander J. Fatiadi at the US National Bureau of Standards (Washington, DC; now the National Institute of Standards and Technology, Gaithersburg, MD), who reported its synthesis in 1977.
Fatiadi prepared croconate violet in 95% yield by treating dipotassium croconate2 or other croconate salts with malononitrile. Recrystallization from water gave crystalline croconate violet as its dihydrate as deep blue needle-like crystals with a metallic appearance. Electron delocaSlization gives the molecule a nearly planar structure.
Heating croconate violet in concentrated hydrochloric acid gave the neutral dihydroxy form as orange crystals that melt with decomposition at 260–270 °C. Despite its color, Fatiadi named this compound “croconic acid violet3”. Dissolving the salt or the acid in water gives a deep violet solution with an intense absorption maximum at 533 nm. The aqueous solution should be handled carefully because it stains the skin.
A recent use of the dyeing properties of croconate violet is protein staining. It is being considered as a replacement for the venerable Coomassie brilliant blue wool dyes4, which have been used for protein staining since 1963. In his original paper, Fatiadi also noted that croconate violet is a semiconductor with an electrical conductivity of 2 x 10–4 S/cm. As of this writing, no hazard information for croconate violet was available.
1. SciFinder: Propanedinitrile, 2,2′-(4,5-dihydroxy-2-oxo-4-cyclopentene-1,3-diylidene)bis-, potassium salt (1:2).
2. CAS Reg. No. 23491-17-0.
3. CAS Reg. No. 66889-00-7.
4. CAS Reg. Nos. 6104-59-2 and 6104-58-1.
Molecules from the journals
Chromium magnesium oxide1 (Cr2MgO4), also known as magnesium chromite, has been known since 1942, when Japanese researcher Hideshi Kuraku used it as a catalyst for methane dehydrogenation. Since then, Cr2MgO4 has been used to catalyze other processes, but more recently, it has been found to have battery applications. This past July, Brian J. Ingram and co-workers at Argonne National Laboratory (Lemont, IL) reported that the substance exhibits unconventional charge-transfer properties and is a promising cathode material in magnesium-ion batteries as an alternative to conventional lithium-ion batteries.
The highly symmetrical hydrocarbon cubane2, Molecule of the Week for March 10, 2008, was synthesized 58 years ago. Last month, the synthesis of its octafluorinated derivative, perfluorocubane3 was reported. The feat was accomplished by Midori Akiyama at the University of Tokyo and her colleagues there and at Hiroshima University, Kyoto University, and AGC Inc. (Tokyo). In addition to the synthesis, the authors demonstrated that the molecule’s unusual electronic structure allows it to absorb an electron inside the cube, resulting in a semistable radical anion.
1. CAS Reg. No. 12053-26-8.
2. CAS Reg. No. 277-10-1.
3. CAS Reg. No. 623570-55-8.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Croconate violet
fast facts
| CAS Reg. No. | 66888-99-1 |
| Empirical formula | C11K2N4O3 |
| Molar mass | 314.34 g/mol |
| Appearance | Deep blue metallic needles (dihydrate) |
| Melting point | Not reported |
| Water solubility | Soluable |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

