What molecule am I?
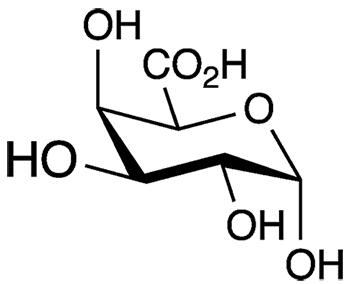
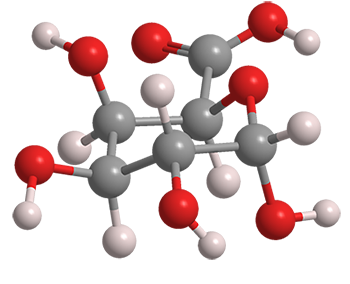
D-Galacturonic acid (GalA) is an oxidized form of the monosaccharide D-galactose1, a component of the disaccharide lactose. Its structure is sometimes displayed in an open, linear form, with a carboxylic acid group on one end of the chain and an aldehyde on the other; but it is more frequently depicted as a closed aldopyranose. This cyclic form can take two configurations: α-D-galacturonic acid, with the hydroxyl adjacent to the ring oxygen in the axial position (shown here), or the β-epimer with the same hydroxyl in the equatorial position.
Why is GalA significant? It is the primary building block and structure-giving element of pectin2 and other biopolymers found throughout the plant kingdom. The polymeric GalA chains in pectin are connected by α-1,4 glycosidic bonds; some of its carboxyl groups are in the form of methyl esters. Pectin molecules have atomic masses of 200,000 g/mol or higher.
According to the Merck Index, pectin is found in the cell walls of all plant tissues and functions as an intercellular connecting material. It is especially abundant (≈30 wt%) in citrus rinds; but it is also commercially sourced from apples, spinach, sugarbeets, and other fruits and vegetables (see photo). Its main use is as a gelling or filling agent in foods such as jellies, jams, desserts, and candies and as a stabilizer in juices and milk-based drinks.
Henri Braconnot at the Royal Academic Society of Nancy (France) first described pectin in 1824. The primary source of commercial GalA is the hydrolysis of pectin, a process that was first reported by Felix Ehrlich3 at the University of Breslau (Germany; now the University of Wrocław [Poland]) in 1917. The process was refined in 2004 by Tetsuya Miyazawa and Toshitaka Funazukuri* at Chuo University (Tokyo). Starting with poly(galacturonic acid) and water with no additives, they used a semibatch flow reactor (220 °C, 10 MPa pressure, 2 min heating time) to obtain a 79% yield of water-soluble products, which were then enzymatically hydrolyzed to GalA and its dimer and trimer.
An internet search showed that worldwide production and consumption of GalA are steadily increasing, but specific numbers are available only in expensive market research reports.
1. CAS Reg. No. 59-23-4.
2. CAS Reg. No. 9000-69-5.
3. Ehrlich was a prominent biochemist. Well before his work on the structure of pectin, he discovered the amino acid isoleucine as a student in 1903.
D-Galacturonic acid hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
MOTW updates
Chlorogenic acid1 (CGA) was the Molecule of the Week for June 10, 2013. An ester formed from caffeic acid and L-quinic acid, it is found in the fruit, leaves, and other tissues of dicotyledonous plants and is an important factor in plant metabolism.
CGA is a known anti-inflammatory agent. In August, Chuqiao Wang, Honggang Fan, and colleagues at Northeast Agricultural University (Harbin, China) and South China Agricultural University (Guangzhou) reported that CGA, along with the polyunsaturated fatty acid derivative resolvin D12, reduced hepatic hemorrhage and inflammatory cell infiltration and had other liver-protective effects in rats that had been subjected to chronic restraint-induced stress.
L-Lysine was the Molecule of the Week for October 18, 2021. It is an essential amino acid that is also valuable as a cocatalyst in a bacterium-driven aldehyde synthesis.
Now lysine has taken on a new role in cancer drug development. For attacking cancer cell proteins, covalent bonding of a drug to protein amino acids is preferable to other forms of attachment because covalent bonding is irreversible and allows the drug to remain longer at the attachment site. Until now, cysteine has been the only amino acid targeted covalently, but it has limited utility because it is relatively rare in cancer proteins.
Recently, scientists at Terremoto Biosciences (San Francisco) began to study lysine as a target. Lysine is attractive because, after cysteine, it is the most nucleophilic amino acid and therefore highly reactive. Terremoto’s work is in the early stages; a practical drug is likely several years off.
1. CAS Reg. No. 202650-88-2.
2. CAS Reg. No. 872993-05-0.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.

D-Galacturonic acid
fast facts
| CAS Reg. No. | 685-73-4 |
| SciFinder nomenclature | D-Galacturonic acid |
| Empirical formula | C6H10O7 |
| Molar mass | 194.14 g/mol |
| Appearance | Hygroscopic white to light yellow crystals or powder |
| Melting point | 166 °C |
| Water solubility | 295 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

