What molecule am I?
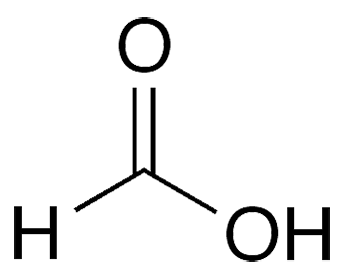
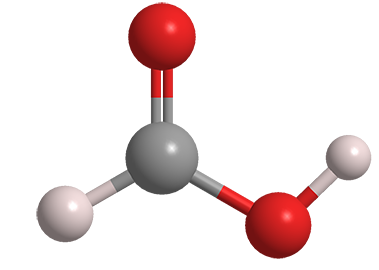
Formic acid is the simplest carboxylic acid. Its discovery in the distillation products of ants is usually attributed to English scientist John Gray in 1671, although there is evidence that a Samuel Fisher made the discovery the year before. The name “formic” comes from formica1, the Latin word for ant and the name of the genus to which many ants belong.
Although ants and other insects produce significant amounts of formic acid, the large worldwide production of the chemical (870 kt in 2021) is made industrially. Most of it is made from carbon monoxide, either by heating it with sodium hydroxide to produce sodium formate, which is then acidified, or via the base-catalyzed reaction of CO and methanol to make methyl formate, which is hydrolyzed to the acid. Formic acid is also a major byproduct of acetic acid manufacture.
Formic acid has a wide range of uses: in leather tanning, as a decalcifier and cleaning product, as a chemical reducing agent, as a preservative in animal feeds, and for manufacturing its salts and esters. Its synthetic method can also be reversed to liberate CO.
This is Earth Week, and this year chemists and students are celebrating it by learning about insect chemistry. Foremost among insect-produced chemicals, formic acid is produced by ants in the subfamily Formicinae (almost 300 species) and some bee species as a venom against predators and as a pheromone to warn fellow insects of danger. Ants in the Formica genus emit formic acid when they bite or spray, causing skin irritation or worse in humans. Fire ants (Solenopsis spp.) cause even more damage because their venom contains toxic alkaloids.
1. This term should not be confused with the commercial plastic laminate Formica, which was so named because it was invented as a substitute for mica in electrical insulation. Formica laminate is not made from formic acid or any of its derivatives.
Formic acid hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
Flammable liquids, category 3 | H226—Flammable liquid and vapor | |
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 1A | H314—Causes severe skin burns and eye damage | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Acute toxicity, inhalation, category 3 | H331—Toxic if inhaled | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule of the future
Triphosphatetrahedrane1 (HCP3) is one of the more unusual compounds to come along in the past year. In October 2021, Martin-Louis Y. Riu, Mengshan Ye, and Christopher C. Cummins* at MIT (Cambridge, MA) reported the synthesis of this tetrahedral molecule, which is isostructural with former Molecule of the Week white phosphorus (P4) and the yet-to-be-synthesized C4H4 hydrocarbon tetrahedrane2.
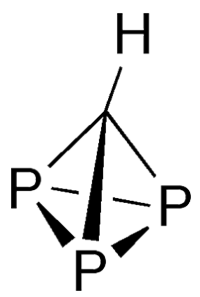
The Cummins team prepared HCP3 via the reaction of bromodichloromethane3 (CHBrCl2) and a triphosphaniobium complex with the help of another niobium complex that strips the halogen atoms from CHBrCl2. HCP3 is stable in dilute tetrahydrofuran solution, but concentrating the solution releases a black precipitate that the authors believe is an HCP3 polymer.
The relative stability of HCP3 is not too surprising considering that its P4 cousin is stable under ambient conditions. According to the authors, the strain energy of the tetrahedral cage is reduced by “the more diffuse frontier orbitals of heavier p-block elements [such as phosphorus] and the propensity for lone pairs to accumulate s orbital character”.
1. CAS Reg. No. 1338340-47-8.
2. CAS Reg. No. 157-39-1.
3. CAS Reg. No. 75-27-4.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
Formic acid fast facts
| CAS Reg. No. | 64-18-6 |
| SciFinder nomenclature | Formic acid |
| Empirical formula | CH2O2 |
| Molar mass | 46.03 g/mol |
| Appearance | Colorless liquid |
| Melting point | 8 °C |
| Boiling point | 101 °C |
| Water solubility | Miscible |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

