What molecule am I?
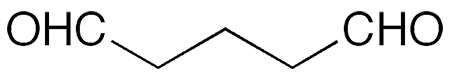
Glutaraldehyde is a dialdehyde and a reduced form of the corresponding diacid, glutaric acid. It was mentioned (as “trimethylene aldehyde”) in the chemical literature as early as 1908, when Russian chemists N. J. Demjanow* and K. Fortunatow synthesized it via the chromic oxide oxidation of 1,5-pentanediol (“trimethylene carbinol”).
Other ways to prepare glutaraldehyde include acidification of 2-methoxy- or 2-ethoxy-3,4-dihydro-2H-pyran and tungstic acid–catalyzed hydrogen peroxide oxidation of cyclopentene.
Because of its highly reactive aldehyde groups, glutaraldehyde is too unstable to store in its pure form. The article of commerce is usually a 25% or 50% aqueous solution; but it is also sold in methanol solution. As the hazard information table shows, it is toxic and must be handled carefully.
Glutaraldehyde’s many uses include sterilizing medical equipment, tanning leather, cross-linking polymers, and acting as a fixative in biochemical procedures. It has even been tried as an ingredient in hair treatments, such as “permanent” waves.
Additional information about glutaraldehyde applications can be found on the ScienceDirect information page.
Glutaraldehyde hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 1B | H314—Causes severe skin burns and eye damage | |
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Acute toxicity, inhalation, category 3 | H331—Toxic if inhaled | |
| Respiratory sensitization, category 1 | H334—May cause allergy or asthma symptoms or breathing difficulties if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Short-term (acute) aquatic hazard, category 1 | H400—Very toxic to aquatic life | |
| Long-term (acute) aquatic hazard, category 1 | H410—Very toxic to aquatic life with long-lasting effects | |
*Data for 50% aqueous solution. This information is from one safety data sheet; some other data sheets contain conflicting information.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
The makaluvamines are natural alkaloids with pyrroloiminoquinone structures. Makaluvamine A1 was isolated from the Fijian sea sponge Zyzzya fuliginosa in 1993 by Chris M. Ireland and coauthors at the University of Utah (Salt Lake City) and American Cyanamid (Pearl River, NY). Two years later, D. John Faulkner and co-workers at the Scripps Institution of Oceanography (La Jolla, CA) isolated makaluvamine K2 from the same sponge species.
Both alkaloids show inhibitory activity toward topoisomerase II3 and cytotoxic activity against HCT-116 human colon cancer cells. In March, John L. Wood and colleagues at Baylor University (Waco, TX) reported the total synthesis of both compounds with a Larock indole synthesis as the first step in each sequence.
Illisimonin A4 is a natural caged sesquiterpenoid with an intricate tricyclo[5.2.1.01,6]decane skeleton. It was isolated in 2017 from the fruit of the southeast Asian shrub Illicium simonsii by Jing Qu, Shan Yu, and co-workers at the Chinese Academy of Medical Sciences and Peking Union Medical College (both in Beijing).
Substances with this structure are known for their high oxidation levels and their neurotrophic (neuron-growing) properties. In March, Markus Kalesse and colleagues at the University of Hanover (Germany) reported a 21-step total asymmetric synthesis of illisimonin A from a previously synthesized propargylic alcohol derivative.
1. CAS Reg. No. 146555-78-4.
2. CAS Reg. No. 174232-36-1.
3. An enzyme that simultaneously cuts both strands of DNA helices and is a frequent chemotherapy target; CAS Reg. No. 142805-56-9.
4. CAS Reg. No. 2162197-76-2.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Glutaraldehyde
fast facts
| CAS Reg. No. | 111-30-8 |
| SciFinder nomenclature | Pentanedial |
| Empirical formula | C5H8O2 |
| Molar mass | 100.12 |
| Appearance | Colorless to light yellow viscous liquid |
| Boiling point | 187–189 °C |
| Water solubility | Miscible |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

