What molecule am I?
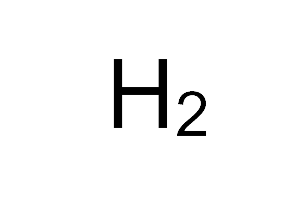
Surprisingly, hydrogen, the smallest and simplest atom and molecule, has not appeared in Molecule of the Week until now. Hydrogen is the most abundant element in the Universe, comprising ≈75% of its mass; but it constitutes only 0.5–1.0 ppm of Earth’s atmosphere. Similarly, hydrogen makes up 0.75 wt% of Earth’s crust; but of course, it is a much larger component of its surface, mostly because of the abundance of water.
English scientist Henry Cavendish first described hydrogen as an element in 1766; he called it “inflammable air”. Seven years later, French chemist Antoine Lavoisier gave it the name hydrogen (“water-former” in Greek). He also showed that there are two hydrogen atoms in the molecule.
Hydrogen is extremely important in industrial chemical manufacture. Because it is hazardous to transport, it is almost always generated at the site where it is used. It is produced mainly by steam-reforming natural gas (CH4 + H2O → CO + 3H2) and other light hydrocarbons. Additional hydrogen is often generated from the carbon monoxide formed during steam reformation via the water–gas shift reaction (CO + H2O → CO2 + H2). Both reactions are catalytic and operate at high temperatures and pressures.
Space does not permit a discussion of all aspects of hydrogen properties and uses. But two recent reports from France are of interest:
- Theoretically, it should be possible to make hydrogen, like all other elements, into a metal, given high enough applied pressure. Many scientists have claimed to make metallic hydrogen, but none have succeeded to the satisfaction of the scientific community. Earlier this year, Paul Loubeyre*, Florent Occelli, and Paul Dumas at the French Alternative Energies and Atomic Energy Commission (Arpajon) used an anvil with a new type of squeezing surface to achieve sufficient pressure (>400 GPa) to create a phase of hydrogen that was identified as a metal by infrared spectroscopy. The result agreed with theoretical calculations of metallicity.
- Hydrogen is one of few energy sources that does not release greenhouse gases when it is burned to generate electricity or propel vehicles. But for now almost all hydrogen production releases CO2 (see above)—about one billion metric tons per year worldwide. According to the International Energy Agency (Paris), renewable energy–powered water electrolysis will not be inexpensive enough to make “green” hydrogen affordable until after 2030.
Hydrogen hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Flammable gases, category 1 | H220—Extremely flammable gas | |
| Gases under pressure, compressed gas | H280—Contains gas under pressure; may explode if heated | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Hydrogen fast facts
| CAS Reg. No. | 1333-74-0 |
| SciFinder nomenclature | Hydrogen |
| Empirical formula | H2 |
| Molar mass | 2.02 g/mol |
| Appearance | Colorless gas |
| Melting point | –252.8 ºC |
| Water solubility | 1.6 mg/L (1 atm, 20 ºC) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

