What molecule am I?
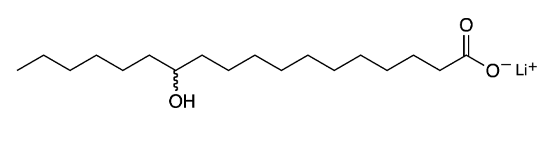
Lithium 12-hydroxystearate is a fatty acid salt commonly known as a “lithium soap”. It is the most common soap used to stabilize and thicken lubricating greases. Lithium salts are generally preferred to soaps with other counterions such as sodium, calcium, and barium.
Recently, Ryan Gordon and Cameron F. Abrams* at Drexel University (Philadelphia) and Spencer T. Stober* at ExxonMobil Research and Engineering (Annandale, NJ) investigated the counterion effects of lithium and sodium 12-hydroxystearate and 12-hydroxystearic acid on the structures of their aggregates, which reflect their rheological properties.
Using quantum mechanical calculations and molecular dynamics simulations, the authors found that the lithium salt formed the most efficiently packed aggregates. This finding is consistent with the compound’s relatively high melting temperature and the high frequency of hydroxyl hydrogen bonding in its aggregates.
According to the authors, these results “may be a factor that makes greases produced from lithium 12-hydroxystearate exhibit higher performance.”
The increasing use of lithium batteries, especially in electric vehicles and electrical power plants, has put pressure on the supply of lithium ores. The resulting unpredictable ore prices have caused some misery to the battery and lithium soap industries.
[Many thanks to Nancy McGuire for assistance with this article—ED].
Lithium 12-hydroxystearate hazard information
| GHS classification*: skin corrosion/irritation, category 2 | |
| H315—Causes skin irritation | |
| GHS classification: serious eye damage/eye irritation, category 2A | |
| H319—Causes serious eye irritation | |
| GHS classification: specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | |
| H335—May cause respiratory irritation | |
**Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Lithium 12-hydroxystearate fast facts
| CAS Reg. No. | 7620-77-1 |
| Empirical formula | C18H35LiO3 |
| Molar mass | 306.42 g/mol |
| Appearance | White crystals or powder |
| Melting point | 200–220 ºC |
| Water solubility | ≈200 mg/L (est.) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

