What molecule am I?
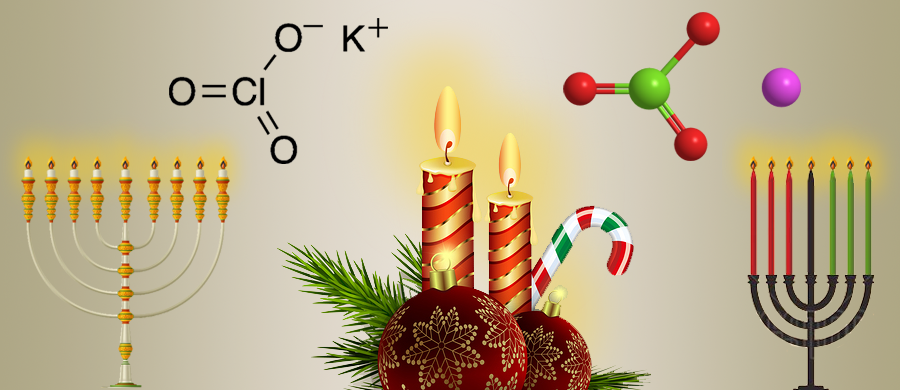
Potassium chlorate (KClO3) is a strong oxidizing agent that has a wide variety of uses. It is or has been a component of explosives, fireworks, safety matches, and disinfectants. As a high school or college chemistry student, you may have used it to generate oxygen in the lab.
Because it is a strong oxidizer, KClO3 must be kept from contacting organic matter; reduced inorganic materials such as elemental sulfur, phosphorus; and iodine; and concentrated acids.
The use of KClO3 in matches dates back to 1826, when English chemist John Walker combined it with antimony(III) sulfide, gum, and starch. When formed into matches, the mixture sometimes (but not always) ignited when struck on sandpaper. Later on, white phosphorus replaced antimony sulfide to make matches more reliable. Eventually, the toxic white phosphorus was superseded by the red allotrope.
Modern safety matches contain no phosphorus; but red phosphorus is embedded in the rough surfaces of matchboxes. Upon striking, the phosphorus ignites, liberating oxygen from the match’s KClO3, which in turn ignites combustible substances (e.g., sulfur) in the matchhead.
It’s the holiday season, so you will be forgiven if you don’t think about all of this chemistry when you light your Christmas, Hanukkah, or Kwanzaa candles.
Potassium chlorate hazard information
| GHS classification*: oxidizing solids, category 1 | |
| H271—May cause fire or explosion; strong oxidizer | |
| GHS classification: acute toxicity, oral, category 4 | |
| H302—Harmful if swallowed | |
| GHS classification: acute toxicity, inhalation, category 4 | |
| H332—Harmful if inhaled | |
| GHS classification: hazardous to the aquatic environment, acute hazard, category 2 | |
| H401—Toxic to aquatic life | |
| GHS classification: hazardous to the aquatic environment, long-term hazard, category 2 | |
| H411—Toxic to aquatic life with long-lasting effects | |
**Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Potassium chlorate
fast facts
| CAS Reg. No. | 3811-04-9 |
| Empirical formula | ClKO3 |
| Molar mass | 122.55 g/mol |
| Appearance | White crystals or powder |
| Melting point | 356–368 ºC (dec.) |
| Water solubility | 80 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

