What molecule am I?
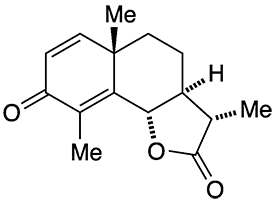
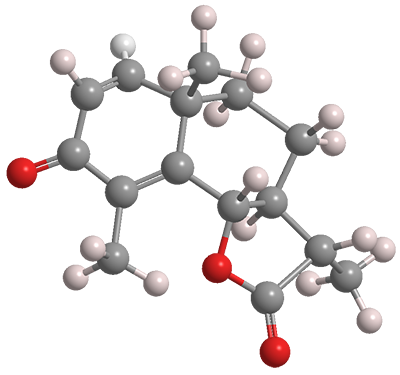
(–)-α-Santonin, often referred to simply as santonin, is compound isolated from the flowers of the Artemisia genus of plants found in Russia and central Asia. It was used as anthelmintic, a drug that kills parasitic worms or removes them from the body. But it has severe side effects (see the hazards information box) and has been replaced by much safer ascaricides.
But santonin lives on in organic synthesis. In a recent report, Phil B. Alper and colleagues at the Genomics Institute of the Novartis Research Foundation (San Diego) describe novel photoinduced rearrangements of 2,5-dienones, including santonin.
The authors found that the rearrangements can be “rerouted” in the presence of amines to give previously unknown structures. When they irradiated tricyclic santonin in the presence of methylamine in a continuous-flow reactor, they obtained unusual [4.4.0] and [5.3.0] fused-ring molecules as major products. Elucidation of the precise mechanisms of the phototransformations is under way.
(–)-α-Santonin hazard information
| GHS classification: acute toxicity, oral, category 4 | |
| H302—Harmful if swallowed | |
| GHS classification: acute toxicity, dermal and inhalation, category 2 | |
| H310/H330—Fatal in contact with skin or if inhaled | |
| GHS classification: skin irritation, category 2 | |
| H315—Causes skin irritation | |
| GHS classification: eye irritation, category 2A | |
| H319—Causes serious eye irritation | |
| GHS classification: specific target organ toxicity—single exposure, category 3, respiratory system | |
| H335—May cause respiratory irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
(–)-α-Santonin fast facts
| CAS Reg. No. | 481-06-1 |
| Molar mass | 264.30 g/mol |
| Empirical formula | C15H18O3 |
| Appearance | Colorless to yellow crystals |
| Melting point | 175 ºC |
| Water solubility | ≈750 mg/L |
MOTW updates
Indigo was the Molecule of the Week for April 23, 2012. It is the oldest dye or pigment used by humans; and the manufactured product is still in use today. Recently, John E. Dauber at the University of California, Berkely, developed a biochemical process to make indigo that is not only environmentally “green”, but it also allows cotton fabric to be treated with an indigo precursor, which then oxidizes in place to dye the fabric.
Previous environmentally problematic Molecules of the Week tetrachloroethylene, N-methyl-2-pyrrolidone, and hexabromocyclododecane are in the news again. They are among the chemicals in the “First 10” list for reevaluation under the revised Toxic Substances Control Act of 2016. But last month the Trump administration put additional restrictions of the substances on hold, with no set date for implementation.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

