What molecule am I?
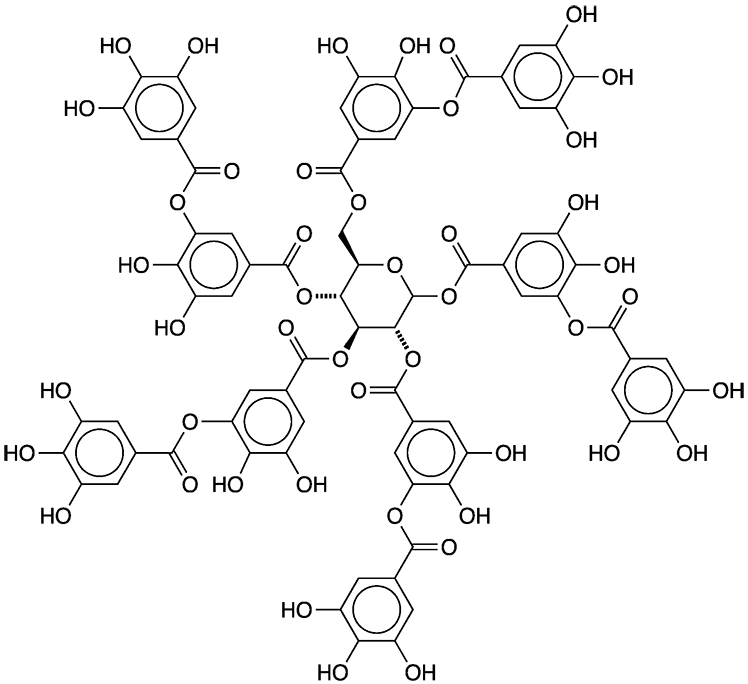
Tannins are polyphenolic biomolecules with carbohydrate backbones that are found in in a wide range of plants. Tannic acid is a specific tannin that formally contains 10 galloyl (3,4,5-trihydroxyphenyl) units surrounding a glucose center. Commercial tannic acid, however, consists of molecules with 2–12 galloyl moieties.
Tannic acid contains no carboxyl groups, but is weakly acidic because of the multiplicity of phenolic hydroxyls. The hydroxyls also cause it to be extremely soluble in water. All regulatory authorities classify it as a nonhazardous substance.
As the name implies, tannins are used in leather tanning. Other commercial uses are in dyeing, ink manufacture, paper sizing, food and wine processing, and production of gallic acid and pyrogallol.
Early reviews of tannins and tannic acid include The Natural Organic Tannins (M. Nierenstein, 1934) and “Gallotannine und Ellagen-gerbstoffe” (O. Th. Schmidt, 1956).
Tannic acid hazard information
| GHS* classification: not a hazardous substance |
MOTW update
Carfilzomib was the Molecule of the Week for December 10, 2012. It is a selective proteasome inhibitor that is used to treat patients with relapsed, refractory multiple myeloma. But recently, data analysis of 24 clinical trials on carfilzomib revealed a high incidence of adverse cardiac events, many of which were severe or life-threatening.
Tannic acid fast facts
| CAS Reg. No. | 1401-55-4 |
| Molar mass | 1701.2 g/mol |
| Empirical formula | C76H52O46 |
| Appearance | Light yellow to tan solid |
| Boiling point | 218 ºC (dec.) |
| Water solubility | 2850 g/L* |
*Some sources report 250 g/L.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

