What molecule am I?
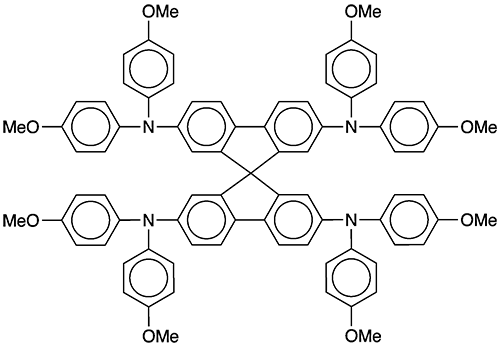
Spiro-OMeTAD1, aka spiro-MeOTAD, is a heavily substituted spirobifluorene derivative that first appeared in the chemical literature in 1998. It was synthesized and characterized by a team led by Donald Lupo at Hoechst Research & Technology (Frankfurt, Germany), Josef Salbeck at the Max Planck Institute for Polymer Research (Mainz, Germany), Michael Grätzel at the Swiss Federal Institute of Technology (Lausanne), and their co-workers.
Patents awarded to these researchers in 1998 claimed uses for spiro-OMeTAD in solar (photovoltaic) cells and radiation detectors. In an article from the same year, Grätzel et al. reported a dye-sensitized heterojunction of titanium dioxide with spiro-OMeTAD, which they described as an “amorphous organic hole-transport material”. A solar cell based on the heterojunction converted photons to electric current in 33% yield—very impressive for that time.
In 2016, Dong Shi, Yuan Li, and coauthors at King Abdullah University of Science and Technology (Thuwal, Saudi Arabia), the Chinese Academy of Sciences (Beijing), and Shanghai Jiao Tong University reported the growth of single crystals of spiro-OMeTAD, in contrast to the amorphous material previously used to form thin films. The charge-carrier transport mobilities of the single-crystal devices were 3 orders of magnitude greater than those that had been achieved by their thin-film counterparts.
Research on the hole-transport properties of spiro-OMeTAD, particularly in perovskite-based solar cells, continues to flourish. More than 550 articles and patents in this field have appeared in 2022 to date.
1. SciFinder: 9,9′-spirobi[9H-fluorene]-2,2′,7,7′-tetramine, N2,N2,N2′,N2′,N7,N7,N7′,N7′-octakis(4-methoxyphenyl)-
Spiro-OMeTAD hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*This information is from one safety data sheet. Other SDSs state “not a hazardous substance or mixture”.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms
Molecule of the Future
Many readers will be familiar with Paxlovid, Pfizer’s combination drug for treating COVID-19 that was covered in the July 4, 2022, Molecules from the journals. Another up-and-coming COVID medication is ensitrelvir1 from Shionogi (Osaka, Japan).
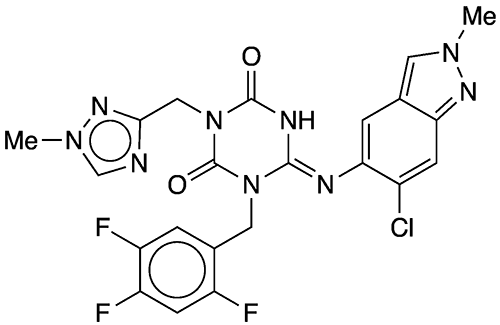
This past March, the research team at Shionogi, led by Yuki Tachibana, announced the discovery of this new antiviral, known at that time as experimental number S-217622. According to this and other reports, ensitrelvir is effective against a wide range of SARS-CoV-2 variants, including Omicron and its subvariants. The same month, Shionogi applied for conditional approval in Japan.
1. CAS Reg. No. 2761992-50-9.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Spiro-OMeTAD fast facts
| CAS Reg. No. | 207739-72-8 |
| Empirical formula | C81H68N4O8 |
| Molar mass | 1225.43 |
| Appearance | White to pale yellow crystals or powder |
| Melting point | 243–248 °C |
| Water solubility | Very slight |
MOTW update
Hydrogen peroxide1 (H2O2) was the Molecule of the Week for January 26, 2007. It is a powerful oxidizing agent with many industrial applications; and it has familiar uses such as an antiseptic for wounds and a reactant in glow sticks. This month, two articles appeared pertaining to the oxygen reduction reaction (ORR) for synthesizing H2O2.
In the first, Curtis P. Berlinguette and several co-workers at the University of British Columbia (Vancouver) and the Canadian Institute for Advanced Research (Toronto) reported a method for directly hydrogenating molecular oxygen without the need for hydrogen gas. They designed a reactor in which hydrogen atoms are electrolytically generated from water in one chamber and then migrate through a palladium foil to a second chamber where they react with incoming oxygen. This process is significantly more efficient than existing indirect hydrogen–oxygen reactor systems.
The second finding was reported by an international team of scientists2, who used density functional theory calculations and experimentation to determine structure–function relationships in the single-atom catalysis of ORRs by cobalt–N4 (CoN4) complexes, in which N4 represents four-nitrogen molecules, chiefly tetrapyrrole and tetrapyridine structures. The authors confirmed that pyrrole-type CoN4 complexes are mainly responsible for the two-electron reduction that produces H2O2, whereas pyridine-type complexes catalyze the four-electron ORR that yields water.
1. CAS Reg. No. 7722-84-1.
2. Mingshan Zhu at Jinan University (Guangzhou, China), Emiliano Cortés at Ludwig Maximilian University of Munich (Germany), Min Liu at Central South University (Changsha, China), and colleagues at these institutions and Hunan University (Changsha) and the National Synchrotron Radiation Research Center (Hsinchu, Taiwan).

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

