What molecule am I?
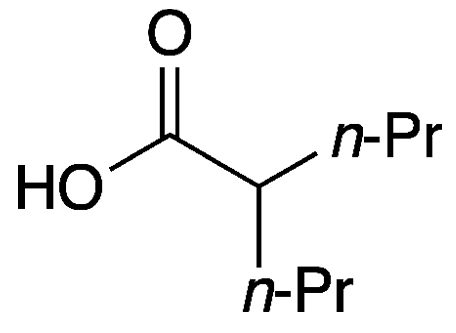
Valproic acid (VPA), formally 2-propylpentanoic acid, is a low–molecular weight branched-chain fatty acid. It is a derivative of valeric (pentanoic) acid1, which occurs naturally in valerian (Valeriana officinalis), an Eastern Hemisphere flowering plant.
VPA has been known since at least the 1880s, when it was synthesized by American chemist Beverly S. Burton. In 1915, a monumental work on optically active fatty acids by Emil Abderhagen and Egon Eichwald at the University of Halle (Germany) reported that VPA was optically active, even though an inspection of its structure shows that that cannot be true.
VPA is widely used for treating several diseases, including epilepsy and bipolar disorder. Until the 1960s, it was used only as a solvent for pharmaceuticals and some industrial chemicals; but in 1963, George Carraz and colleagues at Laboratoire Berthier (Grenoble, France) discovered that all of the compounds they were testing for anticonvulsant activity gave positive results when they were dissolved in VPA. This serendipitous finding led to France’s approval of VPA for treating epilepsy in 1967. The US Food and Drug Administration granted similar approval in 1978.
VPA is well known for inhibiting histone deacetylases, enzymes that allow histone proteins to wrap DNA more tightly than they otherwise would. It also has the ability to reduce the stability of certain proteins, including Cas9 (CRISPR-associated protein 9), which plays an important role in prokaryotic adaptive immunity. Its precise mechanism-of-action for destabilizing Cas9, however, is still largely unknown.
Valproic acid hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Reproductive toxicity, category 1A | H360—May damage fertility or the unborn child | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
MOTW update
Phosphorus pentoxide1 (P4O10) was the Molecule of the Week for September 8, 2008. It is a powerful drying agent that can also be used to remove the elements of water from organic and inorganic compounds.
P4O10 has also found use as a metal-free acid catalyst. In a recent example, Evgeny A. Mostovich and colleagues at Novosibirsk State University (Russia) used it to promote the cyclization of di[aryl(heteroaryl)methyl]malonic acids en route to fused, π-extended spiro[4.4]nonane-1,6-diones, which may have valuable optoelectronic applications.
1. CAS Reg. No. 16752-60-6.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Valproic acid fast facts
| CAS Reg. No. | 99-66-1 |
| SciFinder nomenclature | Pentanoic acid, 2-propyl- |
| Empirical formula | C8H16O2 |
| Molar mass | 144.21 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 222 °C |
| Water solubility | 1.3 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

