Chlorofluorocarbons and Ozone Depletion
En español: Los clorofluorocarbonos y el agujero de ozono
Dedicated at the University of California, Irvine on April 18, 2017.
The language is dry and academic, as is appropriate for the abstract of a scientific paper in the prestigious journal Nature. The research described in the short paper, however, fell like a scientific bombshell, one whose repercussions would be felt around the world. It set off fierce debates, led to a global environmental treaty restricting the use of a broad class of chemicals, and changed the way humans viewed their impact on Earth’s environment. It also led to F. Sherwood Rowland (1927-2012) and Mario J. Molina (*1943) sharing the 1995 Nobel Prize in Chemistry with Paul J. Crutzen of the Max Plank Institute for Chemistry, Mainz, another pioneer in stratospheric ozone research.
Rowland, a professor of chemistry at the University of California, Irvine, and Molina, a postdoctoral fellow in Rowland’s laboratory, had shown that chlorofluorocarbons—CFCs—could destroy ozone, a molecule made up of three oxygen atoms, O3, in Earth’s stratosphere. That stratospheric ozone absorbs ultraviolet radiation that otherwise would reach the surface of Earth. At the time, CFCs were in wide use in refrigeration, air conditioning and aerosol spray cans. The compounds are inert and essentially nontoxic, characteristics that made them well-suited for these applications. These same characteristics, however, also made them a danger to life on Earth.
Commemorative Booklet (PDF)
Landmark Lesson Plan: Chlorofluorocarbons and Ozone Depletion
Widespread use of CFCs
In the 1920s, refrigeration and air conditioning systems used compounds such as ammonia, chloromethane, propane and sulfur dioxide as refrigerants. Though effective, the compounds were toxic and flammable, and exposure to them could result in serious injury or death. A team of chemists at Frigidaire led by Thomas Midgely Jr. (1889-1944) worked to develop nontoxic, nonflammable alternatives to the refrigerants.
The team focused their effort on compounds containing carbon and halogens such as fluorine and chlorine. Such compounds were known to be volatile and chemically inert, both important properties for the team studying their use in refrigeration. The first compound they developed was dichlorodifluoromethane, CCl2F2, which they dubbed “Freon.” Midgely would receive the Society of Chemical Industry’s Perkin Medal for this research in 1937; in 1941, he was awarded the Priestley Medal, the American Chemical Society’s highest award, for his contributions to chemistry.
By the early 1970s, CFCs were in widespread use, and worldwide production of the compounds had reached nearly one million tons per year, representing roughly a $500 million slice of the chemical industry.
Chlorofluoromethanes are being added to the environment in steadily increasing amounts. These compounds are chemically inert and may remain in the atmosphere for 40-150 years, and concentrations can be expected to reach 10 to 30 times present levels. Photodissociation of the chlorofluoromethanes in the stratosphere produces significant amounts of chlorine atoms, and leads to the destruction of atmospheric ozone.”
—F. Sherwood Rowland and Mario J. Molina, Nature, 1974
The importance of ozone
From an environmental standpoint, ozone is a confusing molecule. In the troposphere, the region of the atmosphere from Earth’s surface up to about 6 miles, ozone is a pollutant that is a component of photochemical smog. But in the stratosphere, the region of the atmosphere from 6 to 31 miles, ozone absorbs potentially damaging ultraviolet (UV) radiation.
As the Royal Swedish Academy of Sciences put it in its announcement of the 1995 Nobel Prize in Chemistry: “Even though ozone occurs in such small quantities, it plays an exceptionally fundamental part in life on earth. This is because ozone, together with ordinary molecular oxygen (O2), is able to absorb the major part of the sun’s ultraviolet radiation and therefore prevent this dangerous radiation from reaching the surface. Without a protective ozone layer in the atmosphere, animals and plants could not exist, at least not upon land.”
Rowland’s interest in the fate of CFCs in the atmosphere was sparked by a talk he heard at a conference in 1972. The speaker discussed results obtained by James Lovelock (*1919), a British scientist who had invented a highly sensitive way to measure trace gases. Lovelock had measured trichlorofluoromethane (CFC-11) in the atmosphere in amounts that suggested that practically all of the CFC-11 ever manufactured was still present in the atmosphere.
Rowland decided to devote a portion of his research to understanding the fate of CFCs in the atmosphere. Although CFCs are inert in the lower troposphere, Rowland realized that they can be broken down by UV radiation once they drift up into the stratosphere. In late 1973, Rowland and Molina, who had recently joined Rowland’s lab, used data from a variety of published sources to calculate that CFC molecules released near the surface of Earth would, over decades, wind up in the stratosphere where UV radiation would split off chlorine atoms. Each chlorine atom would react immediately with an ozone molecule, setting off a chain reaction that would destroy thousands of ozone molecules. In their paper, they estimated that if CFC use was banned immediately, ozone loss would go on for years. If CFC production continued, however, ozone loss would be even greater.
“When we realized there was a very effective chain reaction, that changed the CFC investigation from an interesting scientific problem to one that had major environmental consequences,” Rowland told Chemical & Engineering News in an extensive interview in 2007. “You don’t often get many chills down your back when you look at scientific results,” he added, but that had been one of those moments.
From research to resistance
In 1976, the National Academies of Science issued a report affirming the destructive effects of CFCs on stratospheric ozone. Congressional hearings reached similar conclusions, and states and the federal government began exploring bans on the use of CFCs in aerosol cans. The chemical industry maintained that the data on CFCs and stratospheric ozone were inconclusive and didn’t warrant drastic action. When Rowland lectured on CFCs, industry groups often released statements disputing his claims. As Molina recalls today, “Sherry [Rowland] was an established and respected scientist who regularly gave talks all over the world. It seemed that, because of his focus on CFCs and ozone depletion, he started getting fewer invitations to speak. That bothered him.”
Rowland and Molina and the other scientists trying to understand stratospheric chemistry faced serious and fundamental challenges. A significant number of chemical species were clearly involved in the interaction of CFCs and ozone in the stratosphere. Most are highly reactive and present in only trace amounts. Their chemistry was difficult to replicate in the laboratory.
Additionally, stratospheric ozone concentrations fluctuate naturally by geography and by season. The stratosphere is not an easy place to do research in. Measurements of ozone concentration were carried out by instruments carried into the stratosphere by balloons and aircraft. Ozone was also measured by instruments on satellites orbiting Earth, though satellite technology in the mid-1970s was still rather primitive.
All of these uncertainties gave critics of Rowland and Molina’s hypothesis a lot of material to work with. They argued, to many very convincingly, that it simply didn’t make sense to take action against a class of highly useful chemicals on the basis of such flimsy evidence. Industry critics, in particular, argued that it was one thing to propose phasing out the use of CFCs as propellants in aerosol cans—a relatively trivial use of the compounds—but quite another to consider banning their use in refrigerators and air conditioners, where obvious alternatives to CFCs simply didn’t exist at that time.
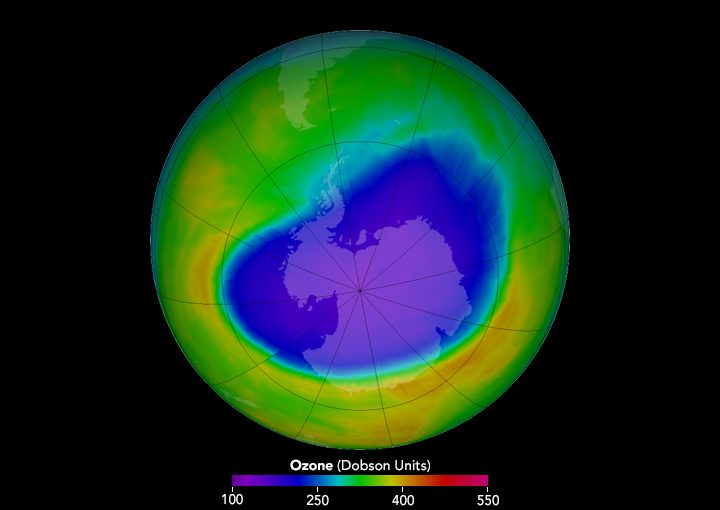
Antarctic ozone hole
The crucial evidence supporting the CFC hypothesis came from British scientists working at the Halley Bay Station of the British Antarctic Survey, who had been taking ground-based measurements of total ozone for decades. In 1984, Joseph C. Farman (1930-2013) and his colleagues at BAS studied the raw data and found that stratospheric ozone had decreased greatly since the 1960s. In 1985, the scientists published an article in Nature announcing that stratospheric ozone over Antarctica was reduced 40% in September, the end of the austral winter.
The Antarctic ozone hole, as it came to be known, made depletion of the ozone layer a real and present danger to lawmakers and the public at large. Predictions of significant increases in the incidence of skin cancer resulting from continued use of CFCs spurred international action. In 1987, 56 countries agreed under what became known as the Montreal Protocol to cut CFC production and use in half. In subsequent years, the protocol was strengthened to require an eventual worldwide phaseout of the production of CFCs and other ozone depleting chemicals.
As a result of Rowland and Molina’s work, humans for the first time realized that their activities could affect Earth’s environment on a planetary scale. As Molina says today, “It doesn’t matter where CFCs are emitted. It is a global problem. What is important is that it led to an international agreement that solved the problem.” The saga of CFCs and the ozone layer holds many lessons for humanity dealing with the even larger challenge of global climate change.
In addition to their Nobel Prize winning work showing that CFCs break down the Earth’s ozone layer, Rowland and Molina were key in convincing scientists, policymakers, and the general public about the harmful effects of CFCs. Their unprecedented advocacy ultimately led to the phasing out of CFCs worldwide through the passage of the Montreal Protocol in 1987. The research of Rowland and Molina brought worldwide attention to the impact of human-contributed pollution on a planetary scale. Their work was among the first to directly effect a global shift in policy, preceding the current debate on climate change.”
—Kenneth C. Janda, professor of chemistry and dean, School of Physical Sciences, University of California, Irvine
Landmark dedication and acknowledgments
Landmark dedication
ACS designated F. Sherwood Rowland and Mario J. Molina’s discovery of CFCs’ harmful effect on ozone as a National Historic Chemical Landmark in a ceremony at the University of California, Irvine, on April 18, 2017. The commemorative plaque reads:
At the University of California, Irvine, F. Sherwood Rowland and Mario J. Molina discovered that chlorofluorocarbons (CFCs) could deplete Earth’s atmospheric ozone layer, which blocks the sun’s damaging ultraviolet rays. When the scientists reported their findings in 1974, CFCs were widely used as refrigerant gases and as propellants in aerosol sprays. Rowland and Molina convinced skeptical industrialists, policymakers, and the public of the danger of CFCs. The scientists’ advocacy — and the discovery by other researchers that the ozone layer over the Antarctic was thinning — led to worldwide phaseout of CFCs and the development of safer alternatives. For their work, Rowland and Molina shared the 1995 Nobel Prize in Chemistry with another atmospheric chemist, Paul J. Crutzen.
Acknowledgments
Adapted for the internet from "Chlorofluorocarbons and Ozone Depletion," produced by the American Chemical Society's National Historic Chemical Landmarks program in 2017.
Additional resources
Lesson Plan
Further reading
- Molina & Rowland 1974 paper "Stratospheric sink for chlorofluorocarbons: chlorine atom-catalysed destruction of ozone" Nature 249 (5460), 810-812
- Mario Molina, Distinguished Professor of Chemistry and Biochemistry, University of California, San Diego
- 1995 Nobel Prize in Chemistry press release
- Montreal Protocol (via EPA.gov)
- Rowland article on research benefiting humanity (via the National Academies)
- Rowland Nobel Lecture in Chemistry, December 8, 1995 (via NobelPrize.org)
- Molina Nobel Lecture in Chemistry, December 8, 1995 (via NobelPrize.org)
- En español: Los clorofluorocarbonos y el agujero de ozono
Cite this page
American Chemical Society National Historic Chemical Landmarks. Chlorofluorocarbons and Ozone Depletion. http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/cfcs-ozone.html (accessed Month Day, Year).


