
By Faith Yarberry and Giovana Stanford
Introduction
Stomachaches are no fun! There is a medicine, patented over 150 years ago and still used today, that reduces stomach acid so that you feel better. This cloudy-looking liquid, called milk of magnesia, is also a key ingredient in this colorful science activity.
Question to Investigate
How does milk of magnesia reduce stomach acid and make us feel better?
Materials
- ¼ cup (60 mL) milk of magnesia (unflavored)
- ½ cup (120 mL) distilled white vinegar
- ¼ cup (60 mL) warm tap water
- 1 or 2 leaves from a head of red cabbage
- 1 quart-size (about 1 liter) resealable or Ziploc plastic bag, storage or freezer type
- 1 tall cup for indicator
- 4 tall clear plastic cups, marked A, B, C, and D
- Spoon to stir (optional)
- Tablespoon and teaspoon
- Paper towels
Additional Safety Requirements
- Water temperature must not exceed 104°F (40°C)
Procedure
Part 1: Tear cabbage leaves and freeze
- Tear two whole cabbage leaves into small pieces (0.5 inch/1 cm each), enough for about 1 cupful (240 mL).
- Place them in a quart-size resealable plastic bag.
- Seal the bag and place it in the freezer for at least one hour (or for a few hours or days).
Part 2: Make cabbage juice indicator
- After at least one hour, open the zip-closing plastic bag with the red cabbage pieces and pour in ¼ cup (60 mL) of warm tap water.
- Squeeze as much air as possible from the bag (without spilling any liquid) as you completely seal it.
- Squish the bag and its contents with your hands for a few minutes until the water becomes a dark purple color.
- Carefully open the bag, tilt, and pour only the “juice” into a tall clear plastic cup, while holding back the cabbage pieces in the bag.
Part 3: Mix it up
- Shake the bottle of milk of magnesia well. Measure and pour 1 tablespoon (tbsp) of milk of magnesia and 1 teaspoon of cabbage juice to each cup.
- Swirl each cup or stir with a spoon to mix the two liquids.
- Add the number of tablespoons of vinegar to each cup as described in the chart and record your observations.
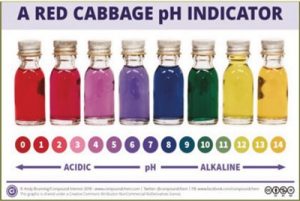
- Compare the final color in each cup to the pH chart on this page and write the corresponding numeral in the chart.
| Cup | Amount of vinegar | Color after adding vinegar | Color after stirring? | Cloudy or clear? | ≈ pH | Acidic, Basic, or Neutral? (Circle one) |
| A | 1 Tbsp | A B N | ||||
| B | 2 Tbsp | A B N | ||||
| C | 3 Tbsp | A B N | ||||
| D | 4 Tbsp | A B N |
How does it work?
The main ingredient in milk of magnesia is magnesium hydroxide. The liquid looks cloudy because only a small amount of the magnesium hydroxide dissolves in water. The rest stays suspended in the liquid, while some sinks to the bottom of the bottle. When you mix vinegar (an acid), with magnesium hydroxide (a base), a chemical reaction happens. The acid and base cancel each other out. The red cabbage juice you added to the mixture changes color during the reaction, letting you know when the vinegar is “used up.” Once all the magnesium hydroxide is used up, the mixture turns clear and remains clear.
How does milk of magnesia help your body? You need acid in your stomach to help you digest your food. However, if the contents of your stomach are too acidic, you may get a stomachache. Milk of magnesia uses up the extra acid and speeds up your digestion so that you feel like yourself again!
Watch a video of how to do this activity:
Faith Yarberry is a Senior Lecturer in Chemistry at the University of Central Arkansas.
Giovana Stanford is an undergraduate student researcher at the University of Central Arkansas.

