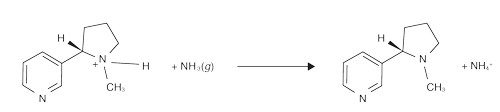Over the summer, the numbers were hard to keep track of. At first, a few dozen mysterious lung illnesses were reported, but by mid-October, the U.S. Centers for Disease Control and Prevention (CDC) said it was investigating about 1,500 cases, including 33 fatalities.
The people affected are mostly in their late teens and early 20s and normally healthy. They reported flu-like symptoms, including vomiting, fatigue, followed by severe shortness of breath. Some of them ended up on ventilators for months. Health investigators determined that the common thread among these cases was vaping.
Pinpointing what specifically causes the illnesses is difficult. Health investigators speculate that a number of factors might be involved, including product contamination, the use of THC (a cannabinoid from marijuana), and device modification. Investigators initially found that the oil vitamin E acetate was in many of the THC products that sick patients had vaped. But a follow-up study examining lung biopsies from some patients didn’t show signs of the oil.
The spike in severe illnesses raised new alarms over e-cigarettes. But even before this summer, public health officials warned that vaping could have unforeseen long-term effects. E-cigarettes haven’t been used long enough, however, for doctors to know what those effects might be.
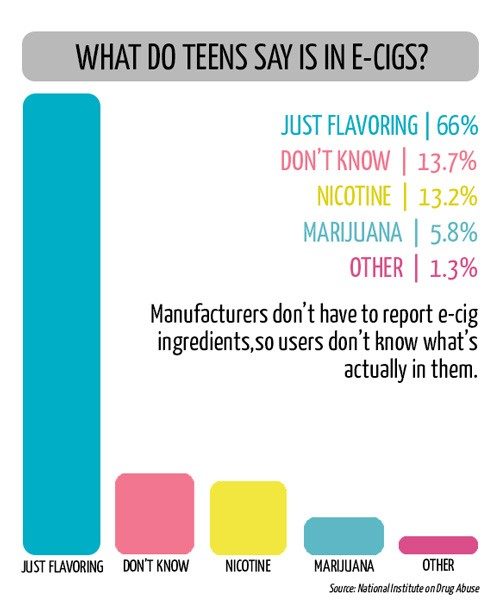
To stem the tide of teen vaping in the United States, sales of e-cigarettes to anyone under 18 became illegal in 2016. Still, the U.S. Food and Drug Administration in 2018 reported that 2 million high school and middle school students had used e-cigarettes regularly during the previous year. In an effort to more effectively prevent teen use, in September Michigan became the first U.S. state to ban flavored e-cigarettes, which are more palatable for young users. A few months before that, San Francisco became the first major U.S. city to ban the sale and distribution of all e-cigarettes. Other communities have also indicated they would take similar action. The CDC urged people to stop vaping.
Public health officials still have a lot to learn about e-cigarettes, but what they do know raises serious concern.
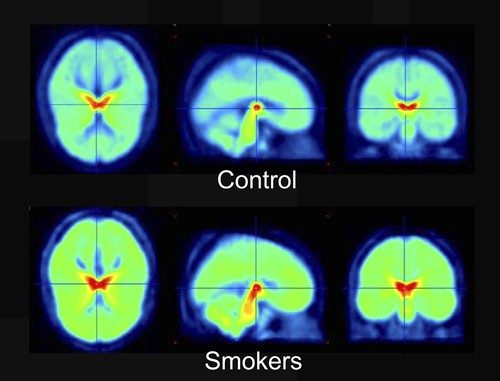
Studies have shown that the density of nicotine receptors—the structures in cell membranes activated by particular molecules—is higher in smokers than in nonsmokers. In these positron emission tomography (PET) scans, the density of nicotine receptors increases as the colors go from blue → green → yellow → red.
Regular versus electronic cigarettes
Smoking traditional cigarettes affects every organ in the body, and is the leading preventable cause of death in the United States: It contributes to nearly 1 out of 5 deaths, according to the CDC. E-cigarettes with nicotine-based liquids are often marketed as an alternative to traditional cigarettes for those addicted to smoking.
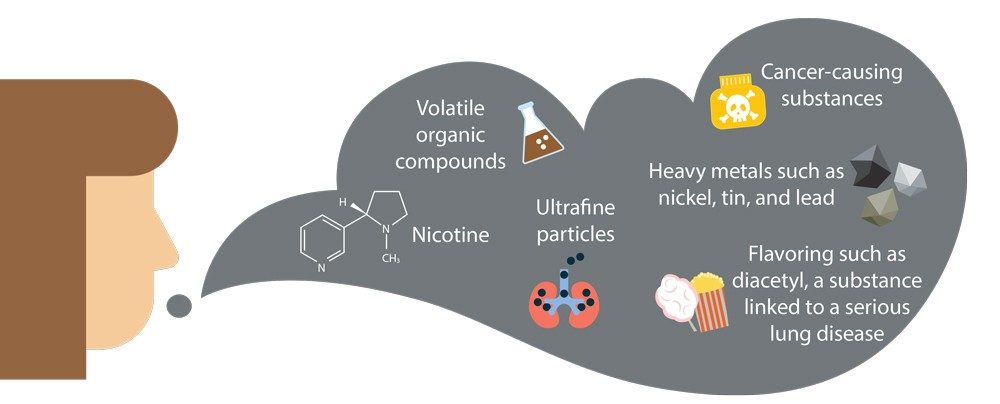
What’s the difference? Well, when lit, tobacco leaves in conventional cigarettes burn and produce smoke containing vaporized nicotine that the user inhales. The smoke also contains thousands of other substances including at least 70 carcinogens, compounds that promote the development of cancer. Capillaries in the lungs absorb the nicotine and other substances, and they enter the bloodstream. The bloodstream carries the absorbed substances to the brain and other parts of the body.
In contrast, electronic cigarettes are tobacco-free. They use a non-combustion method to deliver nicotine to the user. (We’ll focus on nicotine e-cigarettes since they’re in predominant use.) An e-cigarette typically has four parts: a mouthpiece, a rechargeable battery, a cartridge with a liquid, also called a pod, and an atomizer.
The liquid contains nicotine, water, flavoring, and solvents, including glycerin or propylene glycol, that stabilize the mist formed in the atomizer. The atomizer is a little chamber that heats the solution from the cartridge, producing an aerosol mist of tiny liquid droplets (technically not a vapor) that the user inhales. It’s a tiny, nicotine- and flavor-delivering version of a humidifier. When users inhale the mist, their lungs absorb the nicotine and other compounds.
Mist or Vapor?
Despite the term “vaping” that’s associated with e-cigarettes, the devices produce an aerosol mist—not a vapor.
What’s the difference between an aerosol mist and a true vapor?
An aerosol is the suspension of tiny solid or liquid particles in a gas. Clouds, mist, and fog are examples of aerosols. A vapor is the gas phase of a substance that is typically solid or liquid at room temperature. Chlorine, for example, is a gas at room temperature so we refer to Cl2 as chlorine gas, not a vapor. Water, on the other hand, is a liquid at room temperature. So, when water molecules are in the gas phase, we refer to them as water vapor.
The common ingredient
What vaping and tobacco smoking do have in common is nicotine. Nicotine is in a class of compounds known as alkaloids. Alkaloids are organic (carbon-based) molecules that occur naturally in plants. In addition to carbon and hydrogen, alkaloids contain at least one nitrogen atom and usually possess important pharmacological activity, meaning that they act as drugs in the body.
Many modern medicines are either alkaloids derived from plants, or synthetic drugs based on substances originally derived from plants. In addition to nicotine, other well-known alkaloids include caffeine, codeine, cocaine, and morphine.
Examples of Alkaloids
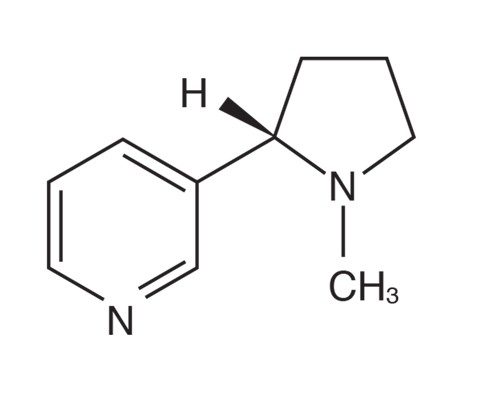
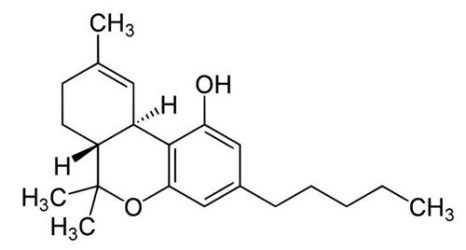
Nicotine, C10H14N2
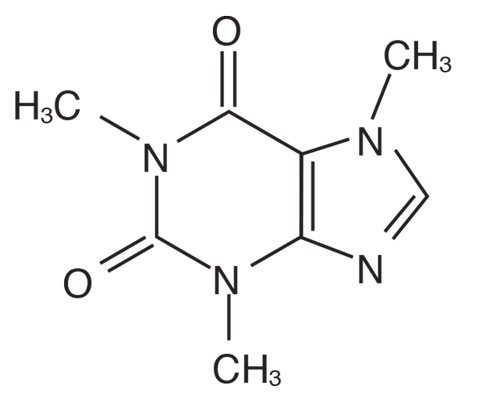
Caffeine, C8H10N4O2
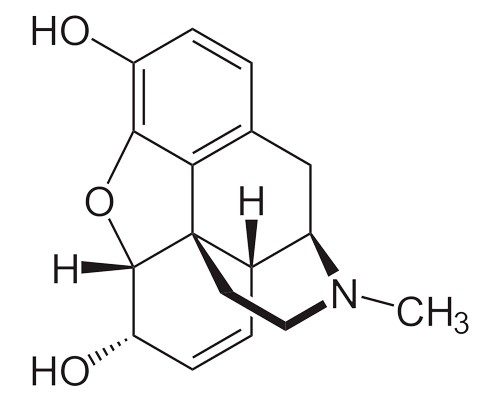
Morphine, C17H19NO3
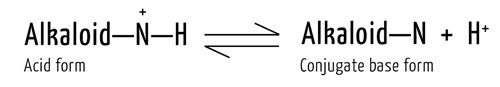
The nitrogen atom(s) in alkaloids give them another important property: They can exist in the acid or conjugate base form. The alkaloid’s nitrogen atoms are basic, meaning the unbonded electrons can accept a proton (H+). In the reverse reaction, H+ is removed from the nitrogen. These reactions occur rapidly in both directions at roughly the same rate, so no overall change in the concentrations of reactants and products is observed:
Nicotine has two nitrogen atoms in its structure, and both can technically be protonated. One becomes protonated only under very acidic conditions, pH < 2, which is an unlikely condition for a consumer product, so let’s focus on the other N.
At a pH above 9, there are more OH– ions than H+ ions present in solution, so the conjugate base form of nicotine would dominate. In acidic solutions, there are a lot more H+ ions. These ions would protonate the nitrogen, making the protonated form of nicotine dominant.
This might seem like a trivial fact, but protonation played an important role in significant legal action taken against tobacco companies decades ago. And recent research suggests that it might have something to do with the market dominance of one particular brand of e-cigarettes, Juul.
Flavor and Formaldehde
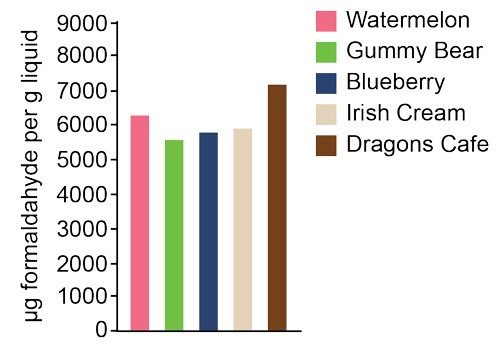
Research has shown that nicotine isn’t the only danger lurking in e-cigarettes. Flavor compounds in e-cigarette liquids break down when heated, forming toxic substances, including formaldehyde. Heating one particular brand in a vaping device produced levels of formaldehyde that were 190 to 270 times the exposure limit determined by the American Conference of Governmental Industrial Hygienists, a nonprofit scientific organization. Formaldehyde can cause coughing, wheezing, and nausea. The U.S. National Toxicology Program recognizes the substance as a known human carcinogen.
Why one proton matters
Starting in the 1990s, multiple states started filing lawsuits against tobacco companies for knowingly hiding information about the dangers of smoking. Company documents that were released as part of these lawsuits revealed that in the 1960s, tobacco companies discovered that the conjugate base form of nicotine was more readily absorbed by the lungs, providing a larger dose of nicotine—and a higher potential for addiction.
Tobacco companies started to add ammonium salts, such as diammonium phosphate [(NH4)2HPO4], to cigarettes. When lit, the salts convert to ammonia (NH3). The ammonia takes protons from acid form nicotine molecules, turning them into the easier-to-absorb, conjugate base form.
The conjugate base form of nicotine feels harsh and scratchy to the back of the throat. The acid form of nicotine is reported to feel milder and smoother when inhaled, but the lungs don’t absorb it as readily.
Researchers who have studied vaping liquids have suggested that this difference in smoothness between acid and conjugate base nicotine might contribute to the popularity of Juul, which first appeared in stores in 2015. Its discreet, thumb-drive-like design, and fruity flavors helped Juul quickly become a top seller. By early August 2019, Juul products accounted for 72% of total e-cigarette sales.
A 2018 study led by David Peyton at Portland State University to evaluate various brands of e-cigarettes found that the two Juul liquids they tested contained mostly acid form (smoother feeling) nicotine.
Peyton and his colleagues also found that Juul pods contained approximately 57 milligrams (mg) of nicotine per milliliter (mL) of solution. Other brands, including Twelve Vapor, Nicquid, and Beard Vape Co., averaged 10 mg of nicotine per mL of solution. Most of the non-Juul brands’ liquids had more than 50% of the harsher, conjugate base form of nicotine.
Peyton and his co-authors see a parallel between the different nicotine forms they found in e-cigarettes and the tobacco companies’ discovery in the 1960s. In an email interview, Peyton noted that “the combination of high-nicotine concentrations and low conjugate base form, like Juul has, will make for a flavor and perception by users that is not harsh, as if the e-cigarette liquid had very little nicotine.”
In the study, however, the quantity of nicotine in the two Juul flavors was higher than in other e-cigarette liquids tested. How much actually gets absorbed by the lungs is another question.
The One Proton Difference
The conjugate base form of nicotine is more readily absorbed by the lungs. To create products with a higher ratio of this form, tobacco companies add ammonium salts to their cigarettes. When the tobacco in a cigarette burns, the heat causes the salts to form ammonia and other compounds. The ammonia reacts with the acidic form of nicotine, removing the proton. This produces more conjugate base form nicotine.
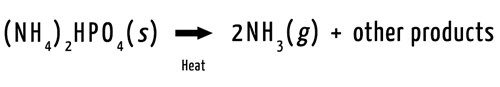
Your brain on nicotine
Research has found that nicotine is bad news, particularly for teenagers, whose brains are still developing.
Among other effects, such as increasing a person’s risk of high blood pressure and diabetes, nicotine boosts the level of dopamine in the brain. Dopamine is a neurotransmitter, a type of chemical released by neurons to send signals to other nerve cells. Dopamine is involved in the activation of the brain’s pleasure and reward centers. Normally, the levels of this neurotransmitter go up when you earn a good grade on a test, win a race, or reach the next level in a computer game. The dopamine makes you feel good about your accomplishment.
When a drug like nicotine artificially causes a dopamine surge, it induces that same positive feeling. The problem is that when the effect wears off, smokers or e-cigarette users want to repeat the experience, so they take more of the drug. This pattern of use and reinforcement can lead to addiction.
This is especially dangerous for teens. Adolescence is a critical period for brain development. Research suggests that nicotine exposure could re-wire teens’ brains with harmful, long-term consequences. For example, it can lower users’ cognitive skills, increase the likelihood that they will try illicit drugs, and increase their risk of developing mental health disorders.
Aside from nicotine, flavorings in vaping liquids could have their own set of health effects. Preliminary lab studies involving human endothelial cells, which line the lungs, have shown that some of the flavored liquids used in e-cigarettes cause significant damage to the cells.
How all of these health factors will play out in the next few years to decades is still unknown. But what recent illnesses and studies on e-cigarettes thus far have shown is that a lot more research is needed to understand the health risks of vaping.
REFERENCES
Duell, A. et al. Free-Base Nicotine Determination in Electronic Cigarette Liquids by 1H NMR Spectroscopy. Chemical Research in Toxicology, May 18, 2018: https://pubs.acs.org/doi/10.1021/acs.chemrestox.8b00097 [accessed Oct 2019].
Nguyen, T. E-Cigarettes’ Chemistry May Explain Their Popularity Among Teens. Chemical & Engineering News, May 24, 2018: https://cen.acs.org/analytical-chemistry/spectroscopy/E-cigaretteschemistry-explain-popularity-among/96/i22 {accessed Oct 2019].
Yuan, M. et al. Nicotine and the Adolescent Brain. The Journal of Physiology, May 27, 2015: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4560573/ [accessed Oct 2019]